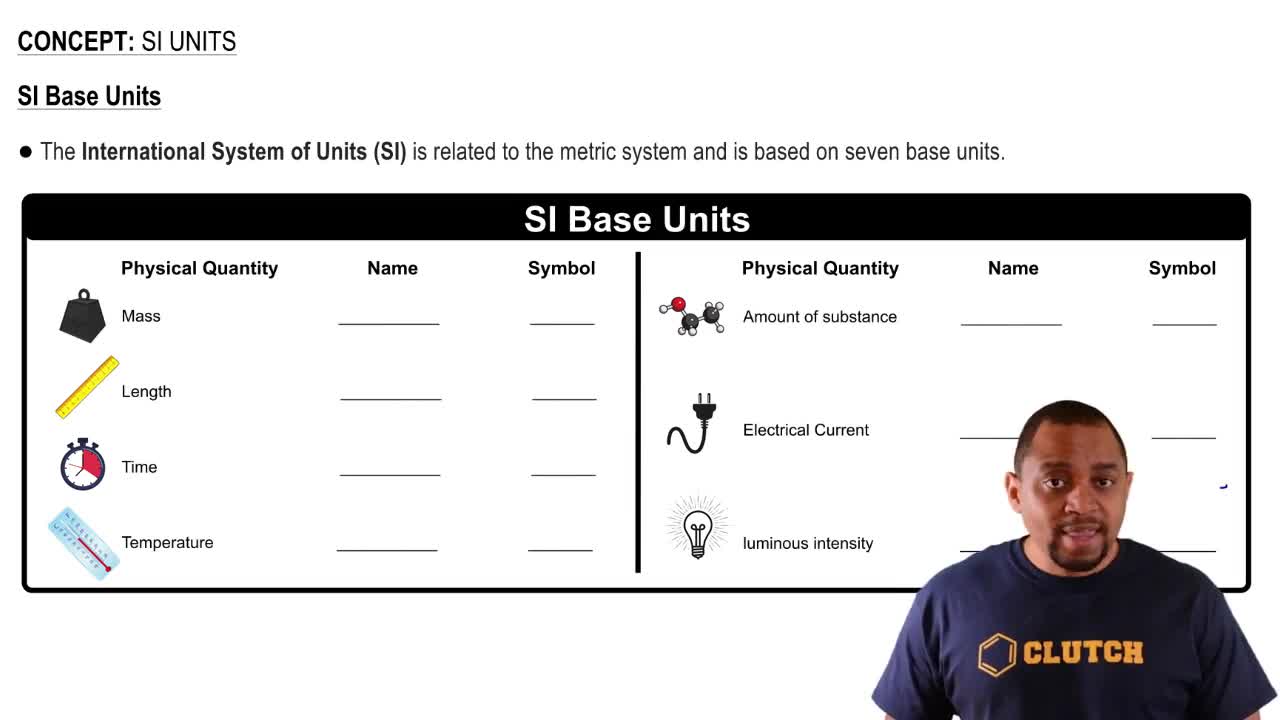
Textbook Question
The height of a horse is usually measured in hands instead of in feet, where 1 hand equals 1/3 ft (exactly). (b) What is the volume in cubic meters of a box measuring 6 ⨉ 2.5 ⨉ 15 hands

 Verified step by step guidance
Verified step by step guidance

The height of a horse is usually measured in hands instead of in feet, where 1 hand equals 1/3 ft (exactly). (b) What is the volume in cubic meters of a box measuring 6 ⨉ 2.5 ⨉ 15 hands
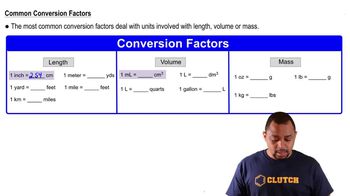
Concentrations of substances dissolved in solution are often expressed as mass per unit volume. For example, normal human blood has a cholesterol concentration of about 200 mg/100 mL. Express this concentration in the following units. (a) mg/L
Which is larger in each pair, and by approximately how much? (a) A liter or a quart