Air pollution in the Mexico City metropolitan area is among the worst in the world. The concentration of ozone in Mexico City has been measured at 441 ppb (0.441 ppm). Mexico City sits at an altitude of 7400 feet, which means its atmospheric pressure is only 0.67 atm. (a) Calculate the partial pressure of ozone at 441 ppb if the atmospheric pressure is 0.67 atm.
Ch.18 - Chemistry of the Environment

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 18, Problem 13
The average concentration of carbon monoxide in the air in an Ohio city in 2006 was 3.5 ppm. Calculate the number of CO molecules in 1.0 L of this air at a pressure of 759 torr and a temperature of 22 °C.
 Verified step by step guidance
Verified step by step guidance1
Convert the temperature from Celsius to Kelvin by adding 273.15 to the Celsius temperature.
Convert the pressure from torr to atmospheres using the conversion factor: 1 atm = 760 torr.
Use the ideal gas law, PV = nRT, to calculate the number of moles of air in 1.0 L. Here, P is the pressure in atm, V is the volume in liters, R is the ideal gas constant (0.0821 L·atm/mol·K), and T is the temperature in Kelvin.
Calculate the number of moles of CO using the concentration in ppm. Since 3.5 ppm means 3.5 molecules of CO per million molecules of air, multiply the total moles of air by 3.5/1,000,000.
Convert the moles of CO to molecules using Avogadro's number, which is approximately 6.022 x 10^23 molecules/mol.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Parts Per Million (ppm)
Parts per million (ppm) is a unit of measurement used to describe the concentration of a substance in a solution or mixture. It indicates how many parts of a substance are present in one million parts of the total mixture. In this context, 3.5 ppm of carbon monoxide means that there are 3.5 molecules of CO for every one million molecules of air.
Recommended video:
Guided course
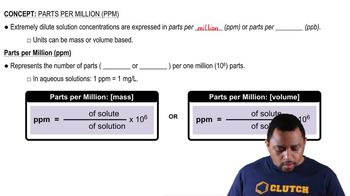
Parts per Million (ppm)
Ideal Gas Law
The Ideal Gas Law is a fundamental equation in chemistry that relates the pressure, volume, temperature, and number of moles of a gas. It is expressed as PV = nRT, where P is pressure, V is volume, n is the number of moles, R is the ideal gas constant, and T is temperature in Kelvin. This law allows us to calculate the number of gas molecules in a given volume under specific conditions.
Recommended video:
Guided course

Ideal Gas Law Formula
Molar Volume of a Gas
The molar volume of a gas is the volume occupied by one mole of an ideal gas at standard temperature and pressure (STP), which is 22.4 liters at 0 °C and 1 atm. However, under different conditions, the molar volume can vary. For calculations involving gas concentrations, knowing the molar volume helps convert between moles and molecules, facilitating the determination of the number of molecules in a specific volume of gas.
Recommended video:
Guided course
The Ideal Gas Law: Molar Mass
Related Practice
Textbook Question
Textbook Question
Air pollution in the Mexico City metropolitan area is among the worst in the world. The concentration of ozone in Mexico City has been measured at 441 ppb (0.441 ppm). Mexico City sits at an altitude of 7400 feet, which means its atmospheric pressure is only 0.67 atm. (b) How many ozone molecules are in 1.0 L of air in Mexico City? Assume T = 25 °C.
5
views
Textbook Question
From the data in Table 18.1, calculate the partial pressures of carbon dioxide and argon when the total atmospheric pressure is 1.05 bar.
Textbook Question
The dissociation energy of a carbon-bromine bond is typically about 276 kJ/mol. (a) What is the maximum wavelength of photons that can cause C-Br bond dissociation?
2
views
