Textbook Question
The dissociation energy of a carbon-bromine bond is typically about 276 kJ/mol. (a) What is the maximum wavelength of photons that can cause C-Br bond dissociation?
2
views

 Verified step by step guidance
Verified step by step guidance
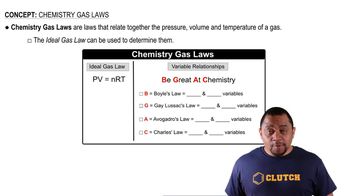
The dissociation energy of a carbon-bromine bond is typically about 276 kJ/mol. (a) What is the maximum wavelength of photons that can cause C-Br bond dissociation?
The wavelength at which the O2 molecule most strongly absorbs light is approximately 145 nm. (a) In which region of the electromagnetic spectrum does this light fall?
The ultraviolet spectrum can be divided into three regions based on wavelength: UV-A (315–400 nm), UV-B (280–315 nm), and UV-C (100–280 nm). (b) In the absence of ozone, which of these three regions, if any, are absorbed by the atmo- sphere?