(b) If a limestone sculpture were treated to form a surface layer of calcium sulfate, would this help to slow down the effects of acid rain? Explain.

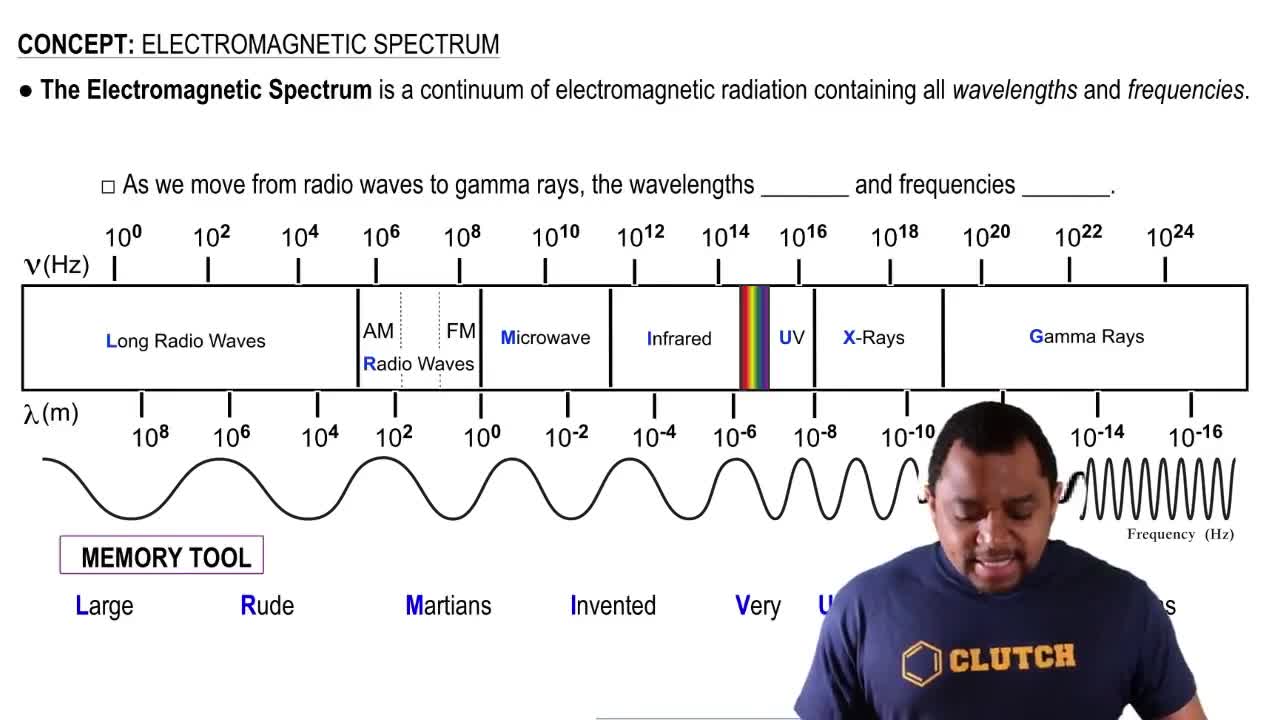
An important reaction in the formation of photochemical smog is the photodissociation of NO : NO2 + hv → NO(g) + O(g) The maximum wavelength of light that can cause this reac- tion is 420 nm. (a) In what part of the electromagnetic spec- trum is light with this wavelength found?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Electromagnetic Spectrum
Photodissociation
Wavelength and Energy Relationship

Alcohol-based fuels for automobiles lead to the production of formaldehyde (CH2O) in exhaust gases. Formaldehyde undergoes photodissociation, which contributes to photo- chemical smog: CH2O + hn ¡ CHO + H The maximum wavelength of light that can cause this reaction is 335 nm. (b) What is the maximum strength of a bond, in kJ/mol, that can be broken by absorption of a photon of 335-nm light?
Alcohol-based fuels for automobiles lead to the production of formaldehyde (CH2O) in exhaust gases. Formaldehyde undergoes photodissociation, which contributes to photo- chemical smog: CH2O + hn ¡ CHO + H The maximum wavelength of light that can cause this reac- tion is 335 nm. (d) Write out the formaldehyde photodis- sociation reaction, showing Lewis-dot structures.
What is the molarity of Na+ in a solution of NaCl whose salinity is 5.6 if the solution has a density of 1.03 g>mL?
Phosphorus is present in seawater to the extent of 0.07 ppm by mass. Assuming that the phosphorus is present as dihydrogenphosphate, H2PO4-, calculate the correspond-ing molar concentration of H2PO4- in seawater.
