Write balanced chemical equations for each of the following reactions: (a) The nitric oxide molecule undergoes photodissociation in the upper atmosphere. (b) The nitric oxide molecule undergoes photoionization in the upper atmosphere. (c) Nitric oxide undergoes oxidation by ozone in the stratosphere.

(a) The EPA threshold for acceptable levels of lead ions in water is 615 ppb. What is the molarity of an aqueous solution with a concentration of 15 ppb?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Parts Per Billion (ppb)
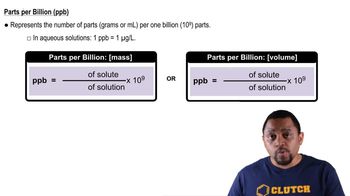
Molarity

Molar Mass

Write balanced chemical equations for each of the following reactions: (d) Nitrogen dioxide dissolves in water to form nitric acid and nitric oxide.
(b) Will Mg(OH)2 precipitate when 4.0 g of Na2CO3 is added to 1.00 L of a solution containing 125 ppm of Mg2+?
(b) Concentrations of lead in the bloodstream are often quoted in units of μg/dL. Averaged over the entire country, the mean concentration of lead in the blood was measured to be 1.6 μg/dL in 2008. Express this concentration in ppb.
In 1986 an electrical power plant in Taylorsville, Georgia, burned 8,376,726 tons of coal, a national record at that time. (a) Assuming that the coal was 83% carbon and 2.5% sulfur and that combustion was complete, calculate the number of tons of carbon dioxide and sulfur dioxide produced by the plant during the year.
The water supply for a midwestern city contains the following impurities: coarse sand, finely divided particulates, nitrate ions, trihalomethanes, dissolved phosphorus in the form of phosphates, potentially harmful bacterial strains, dissolved organic substances. Which of the following processes or agents, if any, is effective in removing each of these impurities: coarse sand filtration, activated carbon filtration, aeration, ozonization, precipitation with aluminum hydroxide?
