(b) Imagine that you could hold two atoms that are bonded together, twist them, and not change the bond length. Would it be easier to twist (rotate) around a single s bond or around a double 1s plus p2 bond, or would they be the same?
Ch.9 - Molecular Geometry and Bonding Theories

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 9, Problem 59a
Propylene, C3H6, is a gas that is used to form the important polymer called polypropylene. Its Lewis structure is (a) What is the total number of valence electrons in the propylene molecule?
 Verified step by step guidance
Verified step by step guidance1
Identify the elements present in propylene: carbon (C) and hydrogen (H).
Determine the number of valence electrons for each type of atom: carbon has 4 valence electrons and hydrogen has 1 valence electron.
Count the number of each type of atom in the propylene molecule: there are 3 carbon atoms and 6 hydrogen atoms.
Calculate the total number of valence electrons by multiplying the number of each type of atom by its valence electrons and summing the results: (3 carbon atoms * 4 valence electrons) + (6 hydrogen atoms * 1 valence electron).
Sum the valence electrons from all the atoms to find the total number of valence electrons in the propylene molecule.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Valence Electrons
Valence electrons are the outermost electrons of an atom and are crucial for determining how atoms bond with each other. In the context of molecules, the total number of valence electrons is calculated by adding the valence electrons of each atom present in the molecule. For propylene (C3H6), this involves considering the contributions from carbon and hydrogen atoms.
Recommended video:
Guided course
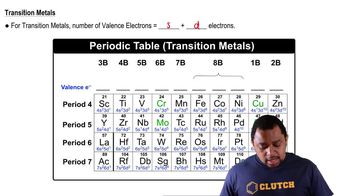
Transition Metals Valence Electrons
Lewis Structure
A Lewis structure is a diagram that represents the bonding between atoms in a molecule and the lone pairs of electrons that may exist. It helps visualize how valence electrons are arranged and shared among atoms. Understanding how to draw and interpret Lewis structures is essential for predicting molecular geometry and reactivity.
Recommended video:
Guided course

Lewis Dot Structures: Ions
Molecular Formula
The molecular formula of a compound indicates the number and type of atoms present in a molecule. For propylene, the molecular formula C3H6 shows that it contains three carbon atoms and six hydrogen atoms. This information is vital for calculating the total number of valence electrons and understanding the molecule's structure and properties.
Recommended video:
Guided course
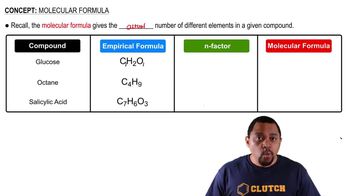
Determining Molecular Formulas
Related Practice
Textbook Question
Textbook Question
(b) What is the hybridization of the carbon atoms in each molecule?
1
views
Textbook Question
Ethyl acetate, C4H8O2, is a fragrant substance used both as a solvent and as an aroma enhancer. Its Lewis structure is
a. What is the hybridization at each of the carbon atoms of the molecule?
Textbook Question
Ethyl acetate, C4H8O2, is a fragrant substance used both as a solvent and as an aroma enhancer. Its Lewis structure is
(c) How many of the valence electrons are used to make s bonds in the molecule?
Textbook Question
Ethyl acetate, C4H8O2, is a fragrant substance used both as a solvent and as an aroma enhancer. Its Lewis structure is
(e) How many valence electrons remain in nonbonding pairs in the molecule?
