Glycine, the simplest amino acid, has the following Lewis structure:
b. What are the hybridizations of the orbitals on the two oxygens and the nitrogen atom, and what are the approximate bond angles at the nitrogen?

 Verified step by step guidance
Verified step by step guidance
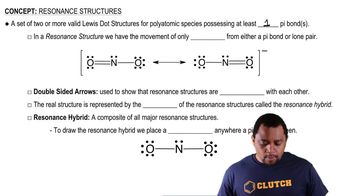

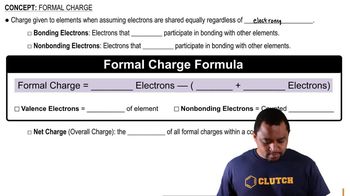
Glycine, the simplest amino acid, has the following Lewis structure:
b. What are the hybridizations of the orbitals on the two oxygens and the nitrogen atom, and what are the approximate bond angles at the nitrogen?
Glycine, the simplest amino acid, has the following Lewis structure:
c. What is the total number of 𝜎 bonds in the entire molecule, and what is the total number of 𝜋 bonds?
d. Would you expect SO3 to exhibit delocalized 𝜋 bonding?
In the formate ion, HCO2-, the carbon atom is the central atom with the other three atoms attached to it. (d) How many electrons are in the p system of the ion?
Consider the following Lewis structure:
a. Does the Lewis structure depict a neutral molecule or an ion? If it is an ion, what is the charge on the ion?
Predict the molecular geometry of each of the following molecules: (b) H O C O C O O H