Predict whether each of the following molecules is polar or nonpolar: (a) IF, (b) CS2, (c) SO3, (d) PCl3, (e) SF6, (f) IF5.
Ch.9 - Molecular Geometry and Bonding Theories

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 9, Problem 43c
Dichloroethylene (C2H2Cl2) has three forms (isomers), each of which is a different substance. (c) How many isomeric forms can chloroethylene, C2H3Cl, have? Would they be expected to have dipole moments?
 Verified step by step guidance
Verified step by step guidance1
Identify the molecular formula of chloroethylene, which is C2H3Cl. This indicates that the molecule contains two carbon atoms, three hydrogen atoms, and one chlorine atom.
Consider the possible arrangements of atoms in the molecule. Since there are two carbon atoms, they can form a double bond, which is characteristic of ethylene derivatives.
Determine the possible positions for the chlorine atom. In chloroethylene, the chlorine atom can be attached to either of the carbon atoms, leading to different structural isomers.
Analyze the geometric arrangement of the atoms. The presence of a double bond between the carbon atoms restricts rotation, allowing for cis-trans isomerism. This means the chlorine atom can be on the same side (cis) or opposite sides (trans) of the double bond.
Evaluate the dipole moments of the isomers. The presence of a chlorine atom, which is more electronegative than carbon and hydrogen, will create a dipole moment. The magnitude and direction of the dipole moment will depend on the spatial arrangement of the atoms in each isomer.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
4mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Isomerism
Isomerism refers to the phenomenon where two or more compounds have the same molecular formula but different structural arrangements or spatial orientations of atoms. In organic chemistry, isomers can be classified into structural isomers, which differ in the connectivity of atoms, and stereoisomers, which have the same connectivity but differ in the arrangement of atoms in space. Understanding isomerism is crucial for predicting the properties and reactivity of compounds.
Recommended video:
Guided course
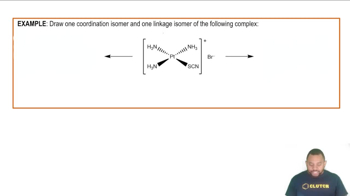
Isomerism in Coordination Complexes Example
Dipole Moment
A dipole moment is a measure of the separation of positive and negative charges in a molecule, indicating its polarity. It arises when there is an uneven distribution of electron density, often due to differences in electronegativity between atoms. Molecules with significant dipole moments are polar and can interact with other polar substances, affecting their physical properties such as solubility and boiling points.
Recommended video:
Guided course
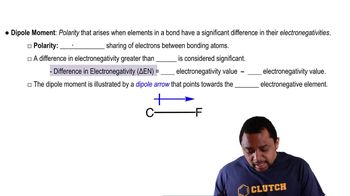
Dipole Moment
Chloroethylene Isomers
Chloroethylene (C2H3Cl) can exist in multiple isomeric forms due to the presence of different arrangements of chlorine and hydrogen atoms around the carbon skeleton. The two main types of isomers for chloroethylene are structural isomers, which include vinyl chloride and other configurations, and geometric isomers, which may arise from restricted rotation around double bonds. The presence of these isomers can influence their chemical behavior and physical properties, including dipole moments.
Recommended video:
Guided course
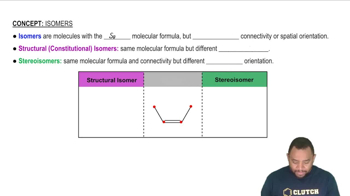
Isomers
Related Practice
Textbook Question
Textbook Question
Predict whether each of the following molecules is polar or nonpolar: (a) CCl4, (b) NH3, (c) SF4, (d) XeF4, (e) CH3Br, (f) GaH3.
Textbook Question
Dichloroethylene (C2H2Cl2) has three forms (isomers), each of which is a different substance. (b) Which of these isomers has a zero dipole moment?
Textbook Question
Dichlorobenzene, C6H4Cl2, exists in three forms (isomers) called ortho, meta, and para:
Which of these has a nonzero dipole moment?
Textbook Question
Draw sketches illustrating the overlap between the following orbitals on two atoms: (a) the 2s orbital on each atom
Textbook Question
Draw sketches illustrating the overlap between the following orbitals on two atoms: (b) the 2pz orbital on each atom (assume both atoms are on the z-axis) (c) the 2s orbital on one atom and the 2pz orbital on the other atom.
2
views
1
rank
