Textbook Question
Consider the following XF4 ions: PF4-, BrF4-, ClF4+, and AlF4-. (c) Which of the ions will have an octahedral electron-domain geometry?
1
views

 Verified step by step guidance
Verified step by step guidance
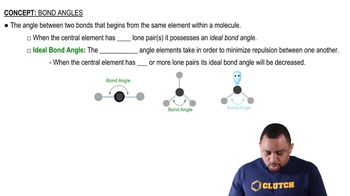
Consider the following XF4 ions: PF4-, BrF4-, ClF4+, and AlF4-. (c) Which of the ions will have an octahedral electron-domain geometry?
Which of the following statements are true?
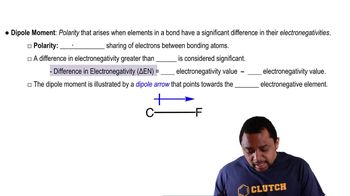
i. The overall dipole moment of a molecule is the vector sum of its individual bond dipoles.
ii. If atoms A and B in an AB𝑛 molecule have different electronegativities, then the AB𝑛 molecule must have a nonzero dipole moment.
iii. The bond dipoles in a tetrahedral AB4 molecule cancel one another.
a. Does SCl2 have a nonzero dipole moment?
(b) It turns out that ozone, O3, has a small dipole moment. How is this possible, given that all the atoms are the same?
(c) Does the molecule BF2Cl have a dipole moment?