The energy-level diagram in Figure 9.40 shows that the sideways overlap of a pair of p orbitals produces two molecular orbitals, one bonding and one antibonding. In ethylene there is a pair of electrons in the bonding orbital between the two carbons. Absorption of a photon of the appropriate wavelength can result in promotion of one of the bonding electrons from the to the molecular orbital. b. Assuming this electronic transition corresponds to the HOMO–LUMO transition, what is the LUMO in ethylene?

A compound composed of 6.7% H, 40.0% C, and 53.3% O has a molar mass of approximately 60 g>mol. (c) What is the geometry and hybridization of the C atom that is bonded to 2 O atoms?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
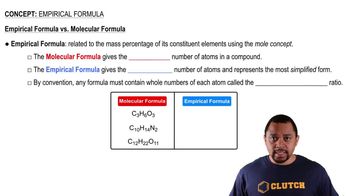
Empirical and Molecular Formulas
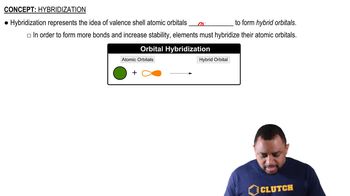
Hybridization
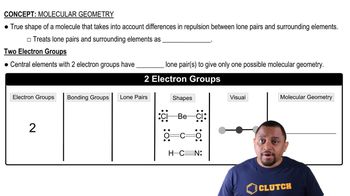
Molecular Geometry
The energy-level diagram in Figure 9.40 shows that the sideways overlap of a pair of p orbitals produces two molecular orbitals, one bonding and one antibonding. In ethylene there is a pair of electrons in the bonding orbital between the two carbons. Absorption of a photon of the appropriate wavelength can result in promotion of one of the bonding electrons from the to the molecular orbital. a. Assuming this electronic transition corresponds to the HOMO–LUMO transition, what is the HOMO in ethylene?
The energy-level diagram in Figure 9.40 shows that the sideways overlap of a pair of p orbitals produces two molecular orbitals, one bonding and one antibonding. In ethylene there is a pair of electrons in the bonding orbital between the two carbons. Absorption of a photon of the appropriate wavelength can result in promotion of one of the bonding electrons from the to the molecular orbital. c. Is the bond in ethylene stronger or weaker in the excited state than in the ground state? Why?
Sulfur tetrafluoride 1SF42 reacts slowly with O2 to form sulfur tetrafluoride monoxide 1OSF42 according to the following unbalanced reaction: SF41g2 + O21g2¡OSF41g2 The O atom and the four F atoms in OSF4 are bonded to a central S atom. (a) Balance the equation.
Sulfur tetrafluoride (SF4) reacts slowly with O2 to form sulfur tetrafluoride monoxide (OSF4) according to the following unbalanced reaction: SF4(g) + O2(g) → OSF4(g) The O atom and the four F atoms in OSF4 are bonded to a central S atom. (b) Write a Lewis structure of OSF4 in which the formal charges of all atoms are zero.
Sulfur tetrafluoride (SF4) reacts slowly with O2 to form sulfur tetrafluoride monoxide (OSF4) according to the following unbalanced reaction: SF4(g) + O2(g) → OSF4(g) The O atom and the four F atoms in OSF4 are bonded to a central S atom. (c) Use average bond enthalpies (Table 8.3) to estimate the enthalpy of the reaction. Is it endothermic or exothermic?
