Draw Lewis structures that satisfy the octet rule for the following molecules and ions: f. HOCl

Write Lewis structures that obey the octet rule for each of the following, and assign oxidation numbers and formal charges to each atom: (a) OCS (b) SOCl2 (S is the central atom) (c) BrO3- (d) HClO2 (H is bonded to O)
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Lewis Structures

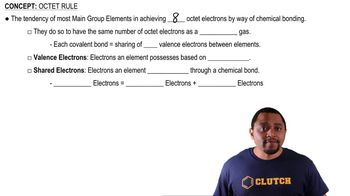
Octet Rule
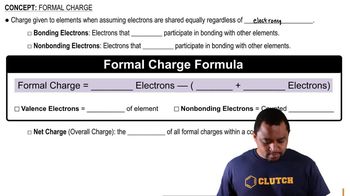
Oxidation Numbers and Formal Charges
Write Lewis structures that satisfy the octet rule for the following molecules and ions: a. NH4+, b. C2F4 (the two C atoms are bonded to one another), c. COCl2 (the Cl atoms are bonded to C), d. HSO3− (H is bonded to one of the O atoms), e. HNC (H and C are both bonded to N), f. ClO3−.
Which one of these statements about formal charge is true? (a) Formal charge is the same as oxidation number. (b) To draw the best Lewis structure, you should minimize formal charge. (c) Formal charge takes into account the different electronegativities of the atoms in a molecule. (d) Formal charge is most useful for ionic compounds. (e) Formal charge is used in calculating the dipole moment of a diatomic molecule.
For each of the following ions of nitrogen and oxygen, write a single Lewis structure that obeys the octet rule, and calculate the oxidation numbers and formal charges on all the atoms: c. NO2+.
(b) With what allotrope of oxygen is it isoelectronic?
Consider the formate ion, HCO2-, which is the anion formed when formic acid loses an H+ ion. The H and the two O atoms are bonded to the central C atom. (b) Are resonance structures needed to describe the structure?
