Energy is required to remove two electrons from Ca to form Ca2+, and energy is required to add two electrons to O to form O2 - . Yet CaO is stable relative to the free elements. Which statement is the best explanation? (a) The lattice energy of CaO is large enough to overcome these processes. (b) CaO is a covalent compound, and these processes are irrelevant. (c) CaO has a higher molar mass than either Ca or O. (d) The enthalpy of formation of CaO is small. (e) CaO is stable to atmospheric conditions.
Ch.8 - Basic Concepts of Chemical Bonding

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 8, Problem 30
List the individual steps used in constructing a Born–Haber cycle for the formation of BaI2 from the elements. Which of the steps would you expect to be exothermic?
 Verified step by step guidance
Verified step by step guidance1

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
5mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Born-Haber Cycle
The Born-Haber cycle is a thermodynamic cycle that relates the lattice energy of an ionic compound to the enthalpy changes involved in its formation from the constituent elements. It provides a systematic way to calculate the lattice energy by considering the various steps, including sublimation, ionization, and electron affinity, that occur when forming an ionic compound from its elements.
Recommended video:
Guided course
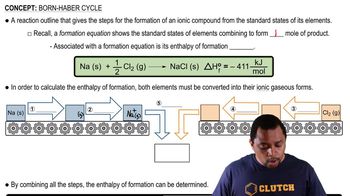
Born Haber Cycle
Lattice Energy
Lattice energy is the energy released when gaseous ions combine to form an ionic solid. It is a crucial factor in determining the stability of ionic compounds. In the context of the Born-Haber cycle, lattice energy is typically a large exothermic value, indicating that the formation of the ionic lattice from gaseous ions is energetically favorable.
Recommended video:
Guided course

Lattice Energy
Enthalpy Changes
Enthalpy changes refer to the heat absorbed or released during a chemical reaction at constant pressure. In the Born-Haber cycle, various steps involve enthalpy changes, such as the enthalpy of sublimation, ionization energy, and electron affinity. Understanding which steps are exothermic (release heat) or endothermic (absorb heat) is essential for predicting the overall energy change in the formation of the compound.
Recommended video:
Guided course

Enthalpy of Formation
Related Practice
Textbook Question
1
views
Textbook Question
Consider the ionic compounds KF, NaCl, NaBr, and LiCl. (a) Use ionic radii (Figure 7.8) to estimate the cation–anion distance for each compound.
Textbook Question
Which of the following trends in lattice energy is due to differences in ionic radii: a. NaCl > RbBr > CsBr, b. BaO > KF, c. SrO > SrCl2?
Textbook Question
Use data from Appendix C, Figure 7.11, and Figure 7.13 to calculate the lattice energy of KI.
Textbook Question
(a) Based on the lattice energies of MgCl2 and SrCl2 given in Table 8.1, what is the range of values that you would expect for the lattice energy of CaCl2?
Textbook Question
(b) Using data from Appendix C, Figure 7.11, Figure 7.13, and the value of the second ionization energy for Ca, 1145 kJ/mol, calculate the lattice energy of CaCl2.
