In the following pairs of binary compounds, determine which one is a molecular substance and which one is an ionic substance. Use the appropriate naming convention (for ionic or molecular substances) to assign a name to each compound: (a) TiCl4 and CaF2 (b) ClF3 and VF3

Draw Lewis structures that satisfy the octet rule for the following molecules and ions: c. SO32−
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Lewis Structures

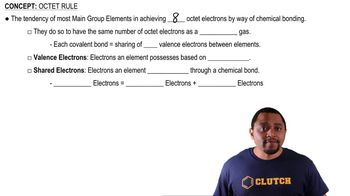
Octet Rule
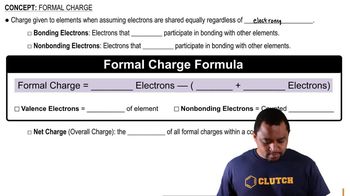
Formal Charge
In the following pairs of binary compounds, determine which one is a molecular substance and which one is an ionic substance. Use the appropriate naming convention (for ionic or molecular substances) to assign a name to each compound: (a) SiF4 and LaF3 (for ionic or molecular substances) to assign a name to each compound: (b) FeCl2 and ReCl6 (c) PbCl4 and RbCl.
In the following pairs of binary compounds, determine which one is a molecular substance and which one is an ionic substance. Use the appropriate naming convention (for ionic or molecular substances) to assign a name to each compound: (c) SbCl5 and AlF3.
Draw Lewis structures that satisfy the octet rule for the following molecules and ions: d. HCN (H and N are both bonded to C),
Draw Lewis structures that satisfy the octet rule for the following molecules and ions: f. HOCl
Write Lewis structures that satisfy the octet rule for the following molecules and ions: a. NH4+, b. C2F4 (the two C atoms are bonded to one another), c. COCl2 (the Cl atoms are bonded to C), d. HSO3− (H is bonded to one of the O atoms), e. HNC (H and C are both bonded to N), f. ClO3−.
