(b) With what allotrope of oxygen is it isoelectronic?

Consider a Lewis structure for SO3 that satisfies the octet rule. Which of the following statements is or are true?
i. SO3 has three equivalent resonance structures.
ii. There are one shorter and two longer S—O bond lengths in SO3.
iii. The S atom in SO3 has a nonzero formal charge.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Lewis Structures

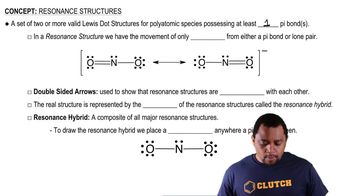
Resonance Structures
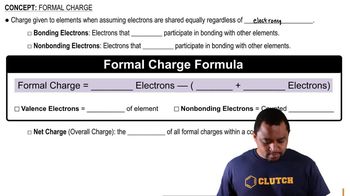
Formal Charge
Consider the formate ion, HCO2-, which is the anion formed when formic acid loses an H+ ion. The H and the two O atoms are bonded to the central C atom. (b) Are resonance structures needed to describe the structure?
Predict the ordering, from shortest to longest, of the bond lengths in CO, CO2, and CO32- .
Which of the following statements about benzene, C6H6, is or are true?
i. Benzene has two equivalent resonance structures.
ii. There are no nonbonding pairs in the Lewis structure for benzene.
iii. Benzene has three short and three long C—C bonds.
b. Which of these compounds or ions is an exception to the octet rule: nitrogen dioxide, borohydride (BH4−), borazine (B3N3H6 which is analogous to benzene with alternating B and N in the ring), or boron trichloride?
