Using only the periodic table as your guide, select the most electronegative atom in each of the following sets: (a) Se, Te, Br, I; (b) Be, Mg, C, Si (c) Al, Si, P, S (d) Zn, Ge, Ga, As.

By referring only to the periodic table, select (d) the element in the group K, C, Zn, F that is most likely to form an ionic compound with Ba.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Ionic Compounds

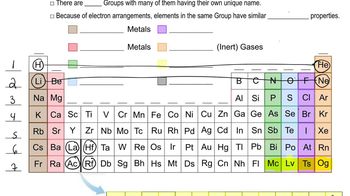
Periodic Table Groups
Barium's Reactivity

Which of the following statements about electronegativity is or are true? i. The alkali metals are the family with the largest electronegativity values. ii. The numerical values for electronegativity have no units. iii. Electronegativity is the ability of an atom in a molecule to attract electron density toward itself.
Place the following pairs of elements in order from smallest to largest difference in electronegativity: K and F, S and O, Br and I, Ca and Se, Li and Cl.
Which of the following bonds are polar: a. B—F, b. Cl—Cl, c. Se—O, d. H—I? Which is the more electronegative atom in each polar bond?
Arrange the bonds in each of the following sets in order of increasing polarity: (a) C—F, O—F, Be—F (b) O—Cl,S—Br, C—P
Arrange the bonds in each of the following sets in order of increasing polarity: (c) C—S, B—F, N—O.
