Textbook Question
Consider the Mg2+, Cl-, K+, and Se2- ions. The four spheres below represent these four ions, scaled according to ionic size. (b) In terms of size, between which of the spheres would you find the (i) Ca2+ and (ii) S2- ions?
1
views


 Verified step by step guidance
Verified step by step guidance
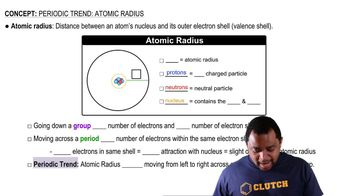
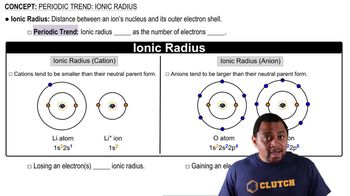

Consider the Mg2+, Cl-, K+, and Se2- ions. The four spheres below represent these four ions, scaled according to ionic size. (b) In terms of size, between which of the spheres would you find the (i) Ca2+ and (ii) S2- ions?
In the following reaction
which sphere represents a metal and which represents a nonmetal?
The graph below shows the ionization energies for a particular element. In which group is the element most likely a member of? [Section 7.3]