(b) Repeat these calculations using Slater’s rules.

The As ¬ As bond length in elemental arsenic is 2.48 Å. The Cl ¬ Cl bond length in Cl2 is 1.99 Å. (a) Based on these data, what is the predicted As ¬ Cl bond length in arsenic trichlo- ride, AsCl3, in which each of the three Cl atoms is bonded to the As atom?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Bond Length

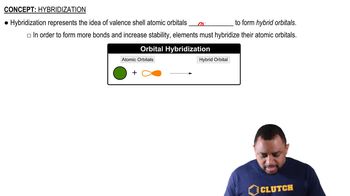
Hybridization
Trends in Bond Lengths
(d) If you remove a single electron from a P atom, which orbital will it come from?
In Table 7.8, the bonding atomic radius of neon is listed as 0.58 Å, whereas that for xenon is listed as 1.40 Å. A classmate of yours states that the value for Xe is more realistic than the one for Ne. Is she correct? If so, what is the basis for her statement?
The following observations are made about two hypothetical elements A and B: The A—A and B—B bond lengths in the elemental forms of A and B are 2.36 and 1.94 Å, respectively. A and B react to form the binary compound AB2, which has a linear structure (that is, ∠B-A-B=180°). Based on these statements, predict the separation between the two B nuclei in a molecule of AB2.
The As ¬ As bond length in elemental arsenic is 2.48 Å. The Cl ¬ Cl bond length in Cl2 is 1.99 Å. (b) What bond length is predicted for AsCl3, using the atomic radii in Figure 7.7?
Elements in group 7A in the periodic table are called the halogens; elements in group 6A are called the chalcogens. (a) What is the most common oxidation state of the chalcogens compared to the halogens?
