Textbook Question
Based on their positions in the periodic table, predict which atom of the following pairs will have the smaller first ionization energy: (a) Br, Kr (b) C, Ca (c) Li, Rb (d) S, Ge (e) Al, B.

 Verified step by step guidance
Verified step by step guidance

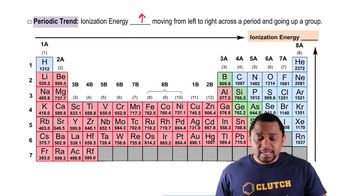
Based on their positions in the periodic table, predict which atom of the following pairs will have the smaller first ionization energy: (a) Br, Kr (b) C, Ca (c) Li, Rb (d) S, Ge (e) Al, B.
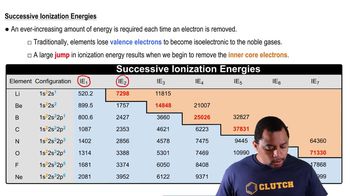
Write equations that show the process for a. the first two ionization energies of lead and b. the fourth ionization energy of zirconium.
Which element has the highest second ionization energy: Li, K, or Be?
Pick the correct word to complete the sentence: The larger the atom, the (smaller/larger) its first ionization energy.
Which element in the periodic table has the largest first ionization energy?