Textbook Question
Consider the stable elements through lead (Z = 82). In how many instances are the atomic weights of the elements out of order relative to the atomic numbers of the elements?

 Verified step by step guidance
Verified step by step guidance
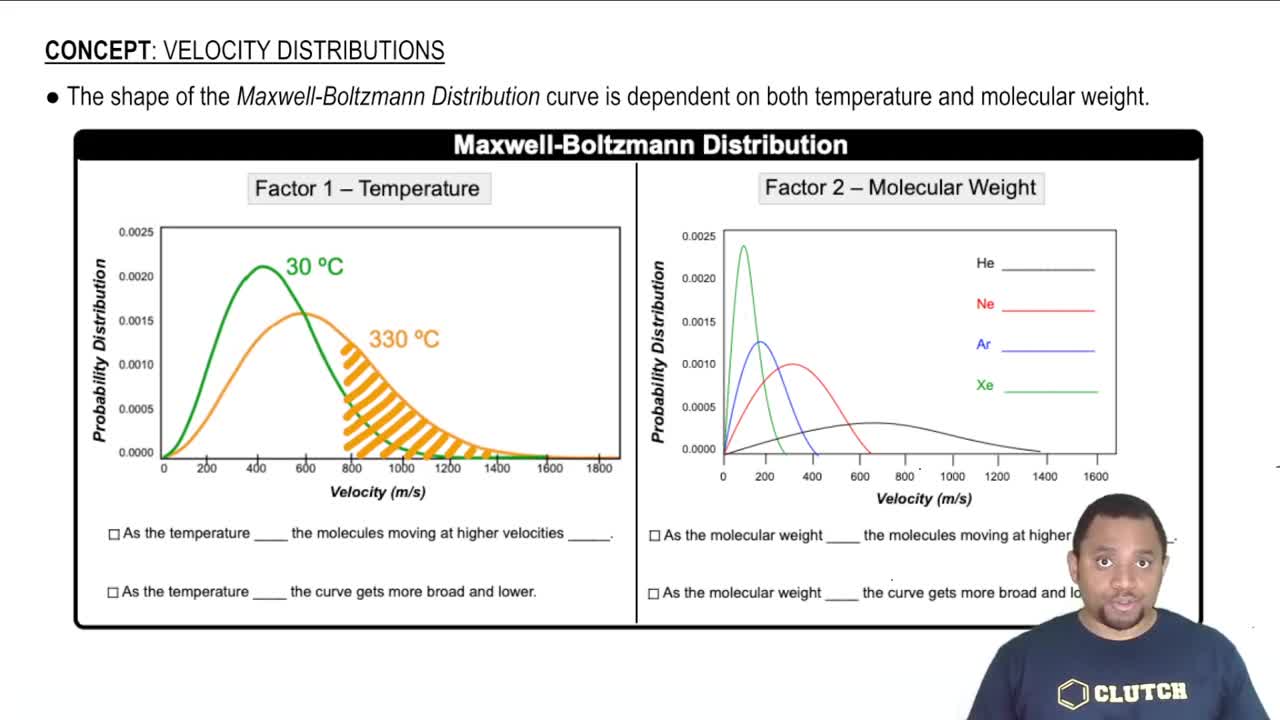
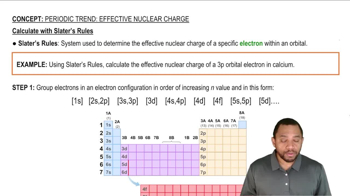
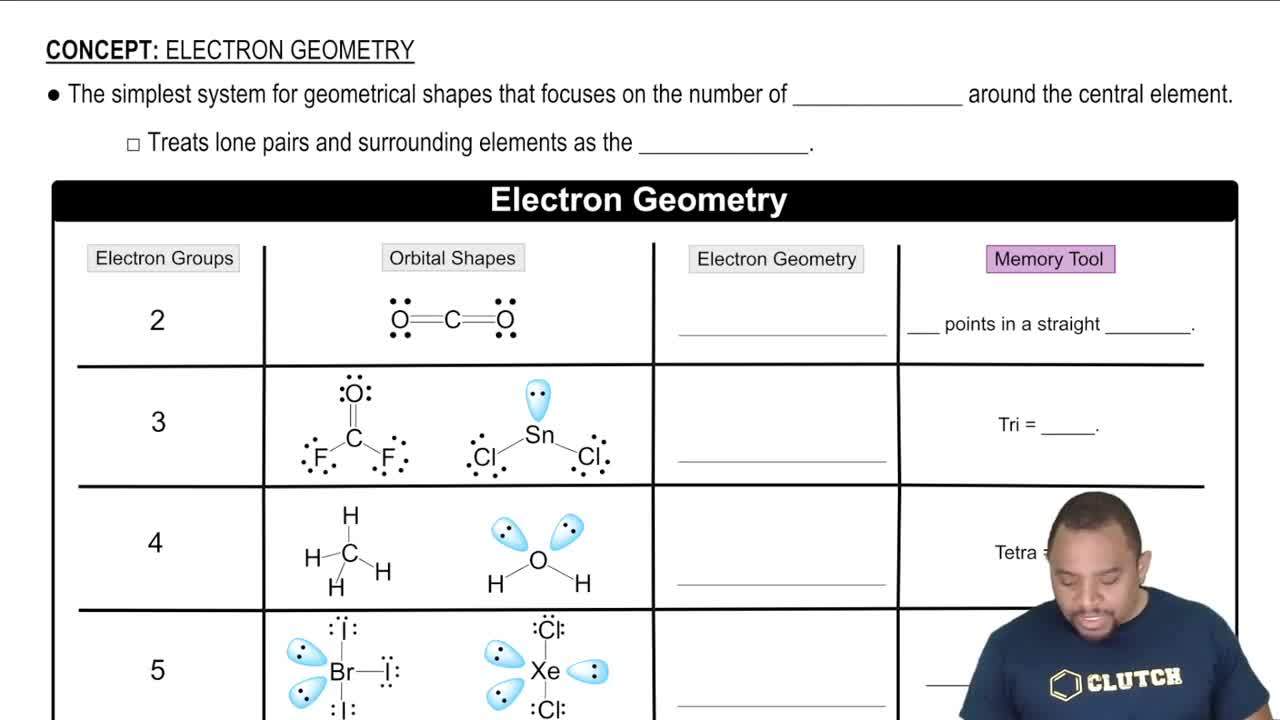
Consider the stable elements through lead (Z = 82). In how many instances are the atomic weights of the elements out of order relative to the atomic numbers of the elements?
Figure 7.4 shows the radial probability distribution functions for the 2s orbitals and 2p orbitals. (a) Which orbital, 2s or 2p, has more electron density close to the nucleus?
(a) If the core electrons were totally effective at screening the valence electrons and the valence electrons provided no screening for each other, what would be the effective nuclear charge acting on the 3s and 3p valence electrons in P?
(b) Repeat these calculations using Slater’s rules.
(d) If you remove a single electron from a P atom, which orbital will it come from?