Is energy emitted or absorbed when the following electronic transitions occur in hydrogen? a. from n = 4 to n = 2

a. Using Equation 6.5, calculate the energy of an electron in the hydrogen atom when n = 2 and when n = 6. Calculate the wavelength of the radiation released when an electron moves from n = 6 to n = 2.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Energy Levels in Hydrogen Atom

Photon Emission and Wavelength
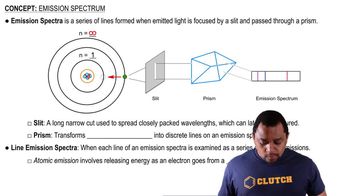
Planck's Constant and the Speed of Light

Is energy emitted or absorbed when the following electronic transitions occur in hydrogen? b. from an orbit of radius 2.12 Å to one of radius 8.46 Å
Indicate whether energy is emitted or absorbed when the following electronic transitions occur in hydrogen: a. from n = 3 to n = 6
Consider a transition of the electron in the hydrogen atom from n = 4 to n = 9. b. Will the light be absorbed or emitted?
The visible emission lines observed by Balmer all involved nf = 2. (a) Which of the following is the best explanation of why the lines with nf = 3 are not observed in the visible portion of the spectrum: (i) Transitions to nf = 3 are not allowed to happen, (ii) transitions to nf = 3 emit photons in the infrared portion of the spectrum, (iii) transitions to nf = 3 emit photons in the ultraviolet portion of the spectrum, or (iv) transitions to nf = 3 emit photons that are at exactly the same wavelengths as those to nf = 2.
The visible emission lines observed by Balmer all involved nf = 2. (b) Calculate the wavelengths of the first three lines in the Balmer series—those for which ni = 3, 4, and 5—and identify these lines in the emission spectrum shown in Figure 6.11.
