The Lyman series of emission lines of the hydrogen atom are those for which nf = 1. (b) Calculate the wavelengths of the first three lines in the Lyman series—those for which ni = 2, 3, and 4.

The hydrogen atom can absorb light of wavelength 1094 nm. (b) Determine the final value of n associated with this absorption.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
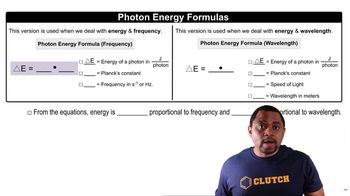
Energy of Photons
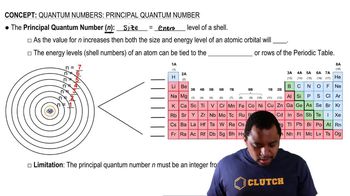
Quantum Energy Levels
Balmer and Rydberg Formulas

One of the emission lines of the hydrogen atom has a wavelength of 93.07 nm. a. In what region of the electromagnetic spectrum is this emission found?
One of the emission lines of the hydrogen atom has a wavelength of 93.07 nm. b. Determine the initial and final values of n associated with this emission.
Order the following transitions in the hydrogen atom from smallest to largest frequency of light absorbed: n = 3 to n = 6, n = 4 to n = 9, n = 2 to n = 3, and n = 1 to n = 2.
Use the de Broglie relationship to determine the wavelengths of the following objects: (a) an 85-kg person skiing at 50 km/hr (b) a 10.0-g bullet fired at 250 m/s
Use the de Broglie relationship to determine the wavelengths of the following objects: (c) a lithium atom moving at 2.5 × 105 m/s (d) an ozone (O3) molecule in the upper atmosphere moving at 550 m/s.
