An AM radio station broadcasts at 1010 kHz, and its FM partner broadcasts at 98.3 MHz. Calculate and compare the energy of the photons emitted by these two radio stations.

One type of sunburn occurs on exposure to UV light of wavelength in the vicinity of 325 nm. (d) These UV photons can break chemical bonds in your skin to cause sunburn—a form of radiation damage. If the 325-nm radiation provides exactly the energy to break an average chemical bond in the skin, estimate the average energy of these bonds in kJ/mol.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Photon Energy
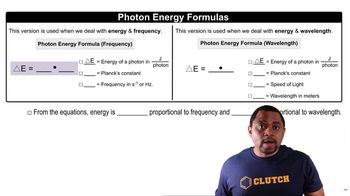
Chemical Bond Energy
Conversion of Energy Units

One type of sunburn occurs on exposure to UV light of wavelength in the vicinity of 325 nm. (c) How many photons are in a 1.00 mJ burst of this radiation?
The energy from radiation can be used to cause the rupture of chemical bonds. A minimum energy of 242 kJ/mol is required to break the chlorine–chlorine bond in Cl2. What is the longest wavelength of radiation that possesses the necessary energy to break the bond? What type of electromagnetic radiation is this?
A diode laser emits at a wavelength of 987 nm. (a) In what portion of the electromagnetic spectrum is this radiation found? (b) All of its output energy is absorbed in a detector that measures a total energy of 0.52 J over a period of 32 s. How many photons per second are being emitted by the laser?
A stellar object is emitting radiation at 3.55 mm. a. What type of electromagnetic spectrum is this radiation? b. If a detector is capturing 3.2×108 photons per second at this wavelength, what is the total energy of the photons detected in 1.0 hour?
