The series of emission lines of the hydrogen atom for which nf = 3 is called the Paschen series. (b) Calculate the wavelengths of the first three lines in the Paschen series—those for which ni = 4, 5, and 6.
Ch.6 - Electronic Structure of Atoms

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 6, Problem 96a
As discussed in the A Closer Look box on 'Measurement and the Uncertainty Principle,' the essence of the uncertainty principle is that we can't make a measurement without disturbing the system that we are measuring. (a) Why can't we measure the position of a subatomic particle without disturbing it?
 Verified step by step guidance
Verified step by step guidance1
Understand that the uncertainty principle is a fundamental concept in quantum mechanics, which states that certain pairs of physical properties, like position and momentum, cannot both be known to arbitrary precision.
Recognize that subatomic particles, such as electrons, exhibit both particle-like and wave-like behavior, making their exact position and momentum inherently uncertain.
Consider that to measure the position of a subatomic particle, we typically use photons or other particles, which interact with the particle being measured.
Realize that this interaction between the measuring photon and the subatomic particle imparts energy to the particle, thus altering its momentum and disturbing its original state.
Conclude that because the act of measurement itself changes the state of the particle, we cannot precisely measure its position without affecting its momentum, illustrating the essence of the uncertainty principle.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Heisenberg Uncertainty Principle
The Heisenberg Uncertainty Principle states that it is impossible to simultaneously know both the exact position and momentum of a particle. This principle arises from the wave-particle duality of matter, where measuring one property inherently disturbs the other due to the nature of quantum mechanics.
Recommended video:
Guided course

Heisenberg Uncertainty Principle
Wave-Particle Duality
Wave-particle duality is a fundamental concept in quantum mechanics that describes how particles, such as electrons, exhibit both wave-like and particle-like properties. When we attempt to measure a particle's position, we treat it as a localized entity, which can alter its momentum and thus disturb its state.
Recommended video:
Guided course
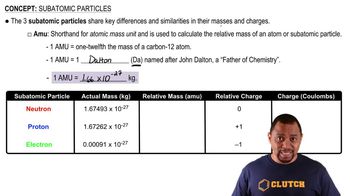
Subatomic Particles
Measurement Disturbance
Measurement disturbance refers to the effect that the act of measuring a quantum system has on that system. In quantum mechanics, the tools used for measurement, such as photons or other particles, can impart energy or momentum to the system, leading to changes in the properties being measured, such as position or velocity.
Recommended video:
Guided course
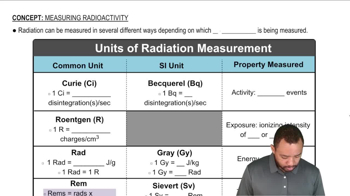
Units of Radiation Measurement
Related Practice
Textbook Question
9
views
Textbook Question
Determine whether each of the following sets of quantum numbers for the hydrogen atom is valid. If a set is not valid, then indicate which of the quantum numbers has a value that is not valid: e. n = 2, l = 2, ml = 1, ms = +1/2
Textbook Question
The Chemistry and Life box in Section 6.7 described the techniques called NMR and MRI. (c) When the 450-MHz photon is absorbed, does it change the spin of the electron or the proton on a hydrogen atom?
Textbook Question
Using the periodic table as a guide, write the condensed electron configuration and determine the number of unpaired electrons for the ground state of d. Sb
