Write a balanced chemical equation for the reaction that occurs when (a) titanium metal reacts with O21g2
Ch.3 - Chemical Reactions and Reaction Stoichiometry

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 3, Problem 19c
Write a balanced chemical equation for the reaction that occurs when (c) the hydrocarbon styrene, C8H81l2, is combusted in air
 Verified step by step guidance
Verified step by step guidance1
Identify the reactants and products in the combustion reaction. The reactants are styrene \( \text{C}_8\text{H}_8 \) and oxygen \( \text{O}_2 \), and the products are carbon dioxide \( \text{CO}_2 \) and water \( \text{H}_2\text{O} \).
Write the unbalanced chemical equation: \( \text{C}_8\text{H}_8 + \text{O}_2 \rightarrow \text{CO}_2 + \text{H}_2\text{O} \).
Balance the carbon atoms first. There are 8 carbon atoms in styrene, so you need 8 \( \text{CO}_2 \) molecules: \( \text{C}_8\text{H}_8 + \text{O}_2 \rightarrow 8\text{CO}_2 + \text{H}_2\text{O} \).
Balance the hydrogen atoms next. There are 8 hydrogen atoms in styrene, so you need 4 \( \text{H}_2\text{O} \) molecules: \( \text{C}_8\text{H}_8 + \text{O}_2 \rightarrow 8\text{CO}_2 + 4\text{H}_2\text{O} \).
Finally, balance the oxygen atoms. Count the total oxygen atoms needed on the right side (8 \( \text{CO}_2 \) gives 16 O, and 4 \( \text{H}_2\text{O} \) gives 4 O, totaling 20 O). Therefore, you need 10 \( \text{O}_2 \) molecules on the left: \( \text{C}_8\text{H}_8 + 10\text{O}_2 \rightarrow 8\text{CO}_2 + 4\text{H}_2\text{O} \).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
4mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Combustion Reactions
Combustion reactions involve the reaction of a substance with oxygen, typically producing heat and light. In organic chemistry, hydrocarbons combust to form carbon dioxide and water. Understanding the general form of combustion reactions is essential for writing balanced equations.
Recommended video:
Guided course
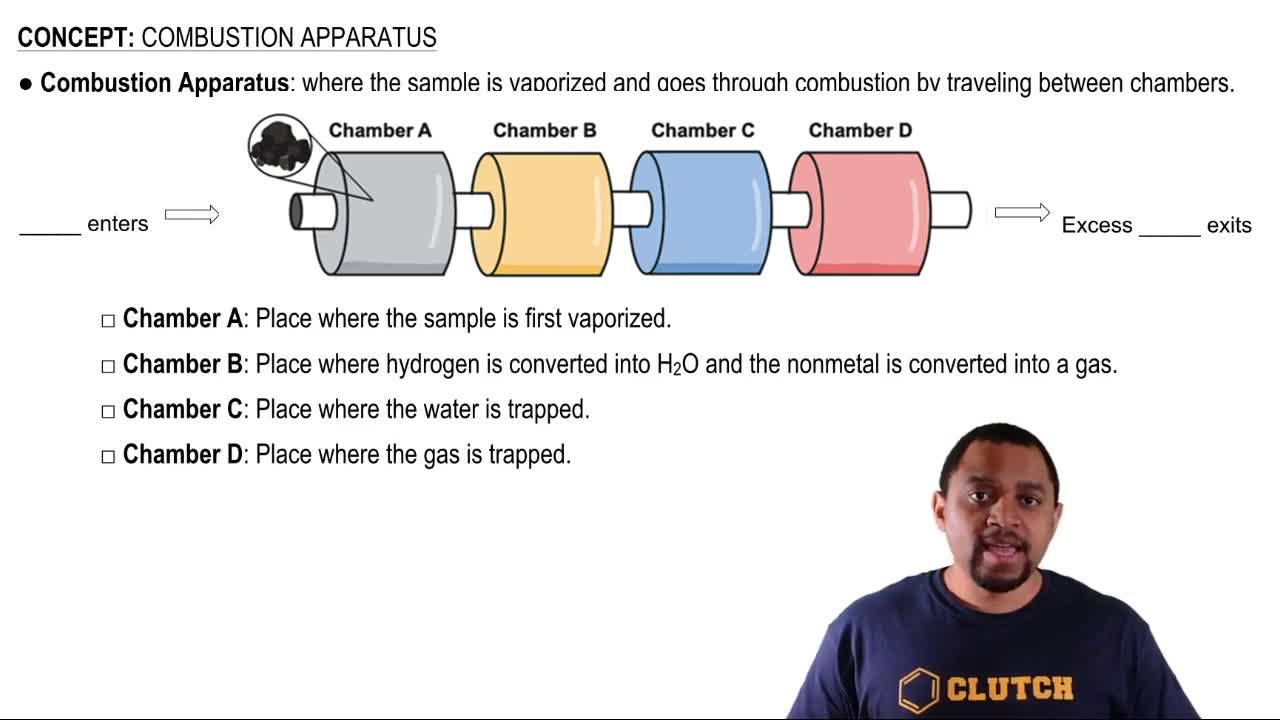
Combustion Apparatus
Balancing Chemical Equations
Balancing chemical equations ensures that the number of atoms for each element is the same on both sides of the equation, adhering to the law of conservation of mass. This process involves adjusting coefficients in front of compounds to achieve balance, which is crucial for accurately representing chemical reactions.
Recommended video:
Guided course

Balancing Chemical Equations
Stoichiometry
Stoichiometry is the quantitative relationship between reactants and products in a chemical reaction. It allows chemists to calculate the amounts of substances consumed and produced, which is vital when balancing equations and predicting the outcomes of reactions, such as combustion.
Recommended video:
Guided course

Stoichiometry Concept
Related Practice
Textbook Question
1
views
Textbook Question
(c) What is the sum of the coefficients in the balanced chemical equation for the combustion of one mole of acetone, C3H6O1l2, in air?
Textbook Question
Write a balanced chemical equation for the reaction that occurs when (a) Mg(s) reacts with Cl2(g)
2
views
Textbook Question
Write a balanced chemical equation for the reaction that occurs when (b) barium carbonate decomposes into barium oxide and carbon dioxide gas when heated
Textbook Question
Write a balanced chemical equation for the reaction that occurs when (d) dimethylether, CH3OCH31g2, is combusted in air.
Textbook Question
Write a balanced chemical equation for the reaction that occurs when (b) silver(I) oxide decomposes into silver metal and oxygen gas when heated (c) propanol, C3H7OH(l) burns in air (d) methyl tert-butyl ether, C5H12O(l), burns in air.
