(a) What is the mass, in grams, of one mole of 12C?

Without doing any detailed calculations (but using a periodic table to give atomic weights), rank the following samples in order of increasing numbers of atoms: 42 g of NaHCO3,1.5molCO2,6.0×1024 Ne atoms.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
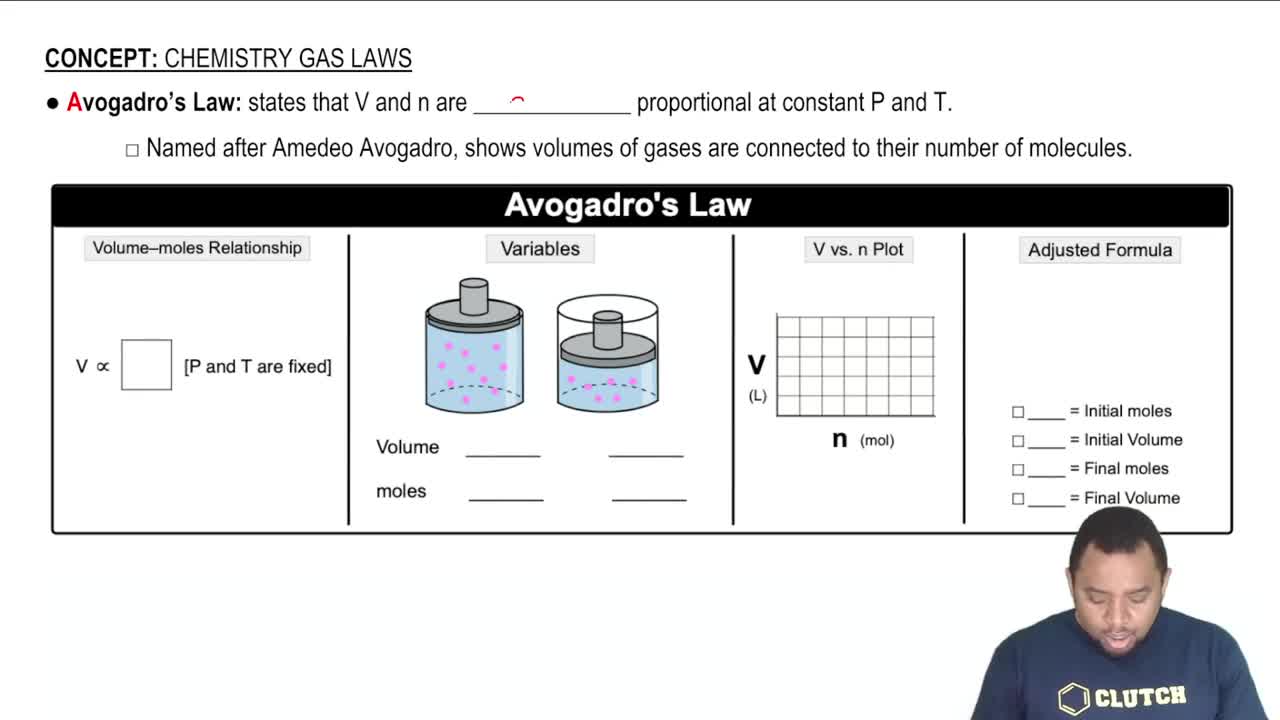
Key Concepts
Molar Mass

Avogadro's Number
Stoichiometry

(b) How many bromine atoms are present in one mole of 12C?
Without doing any detailed calculations (but using a periodic table to give atomic weights), rank the following samples in order of increasing numbers of atoms: 0.50 mol H2O, 23 g Na, 6.0⨉1023 N2 molecules.
What is the mass, in kilograms, of an Avogadro's number of people, if the average mass of a person is 160 lb?
What is the mass, in kilograms, of an Avogadro's number of people, if the average mass of a person is 160 lb? How does this compare with the mass of Earth, 5.98⨉1024 kg?
If an Avogadro’s number of pennies is divided equally among the 321 million men, women, and children in the United States, how many dollars would each receive? How does this compare with the gross domestic product (GDP) of the United States, which was \$21.4 trillion in 2019? (GDP is the total market value of the nation’s goods and services.)
