A mixture of N21g2 and H21g2 reacts in a closed container to form ammonia, NH31g2. The reaction ceases before either reactant has been totally consumed. At this stage 3.0 mol N2, 3.0 mol H2, and 3.0 mol NH3 are present. How many moles of N2 and H2 were present originally?

When a mixture of 10.0 g of acetylene (C2H2) and 10.0 g of oxygen (O2) is ignited, the resulting combustion reaction produces CO2 and H2O. (c) How many grams of CO2 and H2O are present after the reaction is complete?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
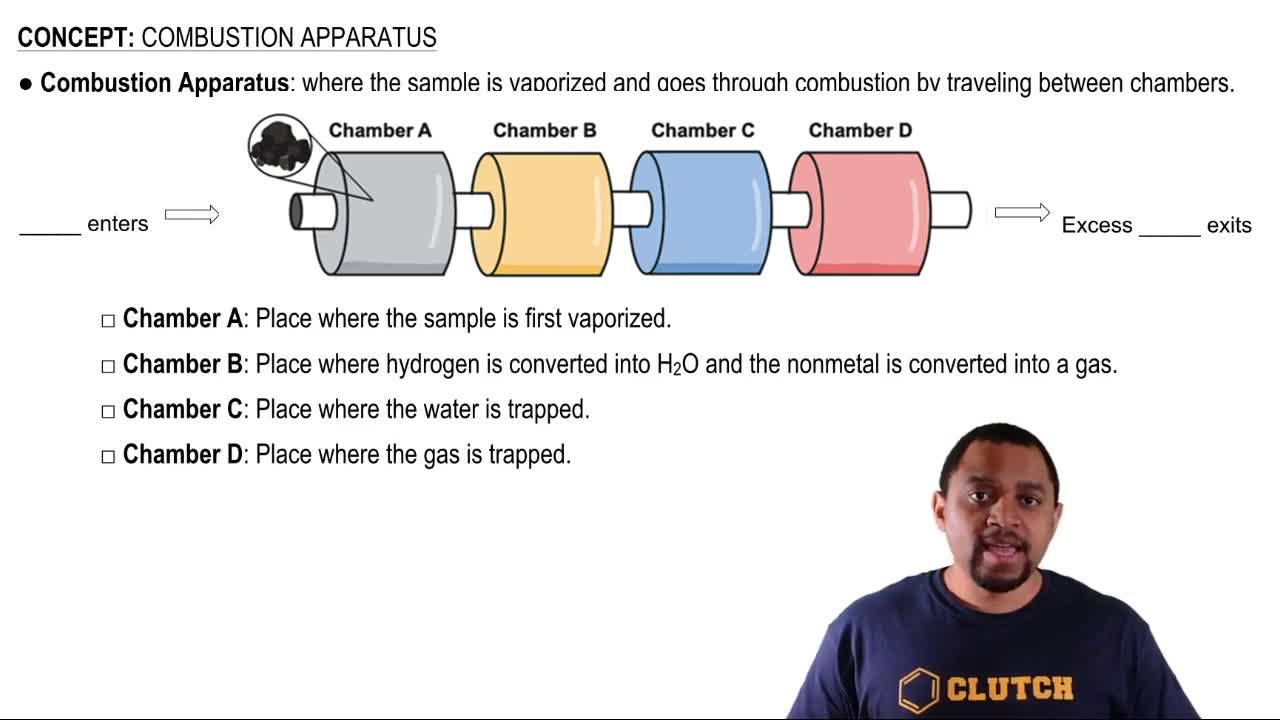
Stoichiometry

Combustion Reactions
Molar Mass

A mixture containing KClO3, K2CO3, KHCO3, and KCl was heated, producing CO2, O2, and H2O gases according to the following equations: 2 KClO31s2¡2 KCl1s2 + 3 O21g2 2 KHCO31s2¡K2O1s2 + H2O1g2 + 2 CO21g2 K2CO31s2¡K2O1s2 + CO21g2 The KCl does not react under the conditions of the reaction. If 100.0 g of the mixture produces 1.80 g of H2O, 13.20 g of CO2, and 4.00 g of O2, what was the composition of the original mixture? (Assume complete decomposition of the mixture.) How many grams of K2CO3 were in the original mixture?
When a mixture of 10.0 g of acetylene (C2H2) and 10.0 g of oxygen (O2) is ignited, the resulting combustion reaction produces CO2 and H2O. (c) How many grams of C2H2 are present after the reaction is complete?
The source of oxygen that drives the internal combustion engine in an automobile is air. Air is a mixture of gases, principally N2(79%) and O2(20%). In the cylinder of an automobile engine, nitrogen can react with oxygen to produce nitric oxide gas, NO. As NO is emitted from the tailpipe of the car, it can react with more oxygen to produce nitrogen dioxide gas. (b) Both nitric oxide and nitrogen dioxide are pollutants that can lead to acid rain and global warming; collectively, they are called 'NOx' gases. In 2009, the United States emitted an estimated 19 million tons of nitrogen dioxide into the atmosphere. How many grams of nitrogen dioxide is this?
The source of oxygen that drives the internal combustion engine in an automobile is air. Air is a mixture of gases, principally N2(79%) and O2(20%). In the cylinder of an automobile engine, nitrogen can react with oxygen to produce nitric oxide gas, NO. As NO is emitted from the tailpipe of the car, it can react with more oxygen to produce nitrogen dioxide gas. (c) The production of NOx gases is an unwanted side reaction of the main engine combustion process that turns octane, C8H18, into CO2 and water. If 85% of the oxygen in an engine is used to combust octane and the remainder used to produce nitrogen dioxide, calculate how many grams of nitrogen dioxide would be produced during the combustion of 500 g of octane.
