The molecular formula of allicin, the compound responsible for the characteristic smell of garlic, is C6H10OS2. (d) How many S atoms are present in 5.00 mg of allicin?

The molecular formula of aspartame, the artificial sweetener marketed as NutraSweet®, is C14H18N2O5. (d) How many hydrogen atoms are present in 1.00 mg of aspartame?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
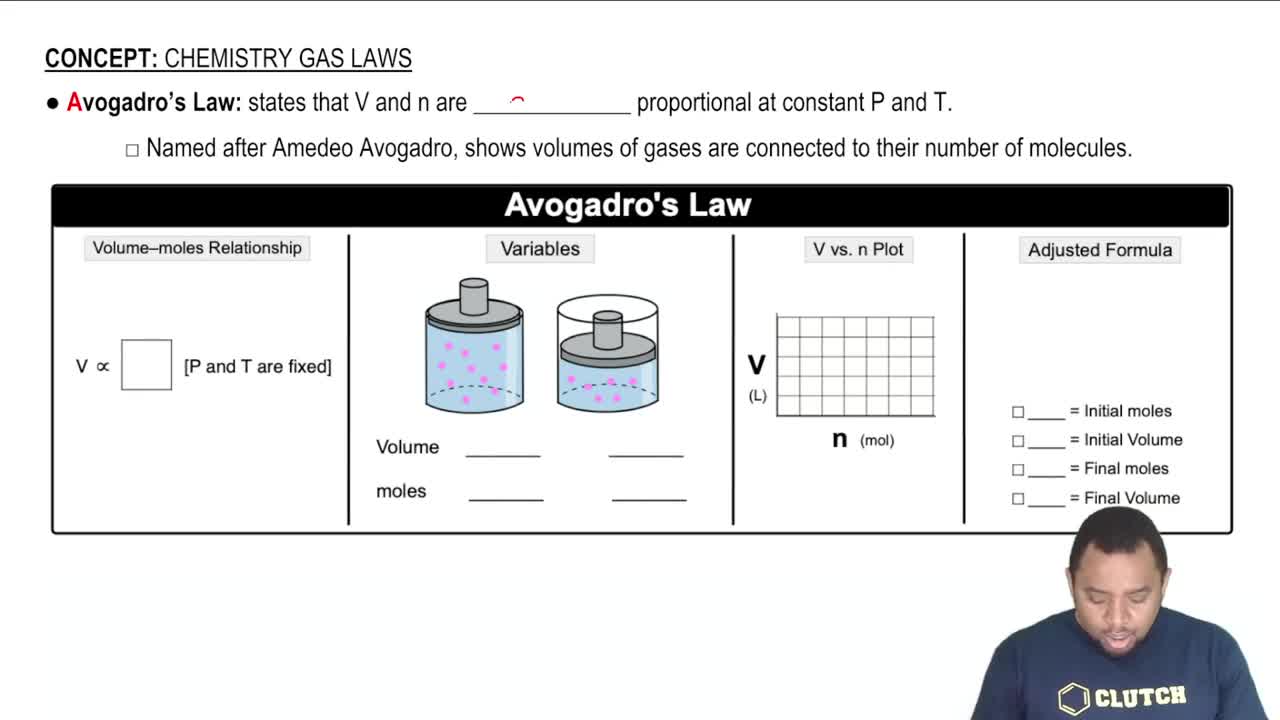
Key Concepts
Molecular Formula

Molar Mass

Avogadro's Number
The molecular formula of aspartame, the artificial sweetener marketed as NutraSweet®, is C14H18N2O5. (a) What is the molar mass of aspartame?
The molecular formula of aspartame, the artificial sweetener marketed as NutraSweet®, is C14H18N2O5. (c) How many molecules of aspartame are present in 1.00 mg of aspartame?
A sample of glucose, C6H12O6, contains 1.250×1021 carbon atoms. (c) How many moles of glucose does it contain?
A sample of the male sex hormone testosterone, C19H28O2, contains 3.88×1021 hydrogen atoms. (b) How many molecules of testosterone does it contain?
The allowable concentration level of vinyl chloride, C2H3Cl, in the atmosphere in a chemical plant is 2.0×10−6 g/L. How many moles of vinyl chloride in each liter does this represent?
