Calculate the percentage by mass of the indicated element in the following compounds: c. hydrogen in ammonium sulfate, (NH4)2SO4, a substance used as a nitrogen fertilizer
Ch.3 - Chemical Reactions and Reaction Stoichiometry

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 3, Problem 26f
Calculate the percentage by mass of the indicated element in the following compounds: f. carbon in capsaicin, C18H27NO3, the compound that gives the hot taste to chili peppers.
 Verified step by step guidance
Verified step by step guidance1
Step 1: First, we need to calculate the molar mass of capsaicin, C18H27NO3. The molar mass of an element is the mass of one mole of that element. It is calculated by adding up the atomic masses of all the atoms in the compound. The atomic masses of carbon (C), hydrogen (H), nitrogen (N), and oxygen (O) are approximately 12.01 g/mol, 1.01 g/mol, 14.01 g/mol, and 16.00 g/mol, respectively.
Step 2: Multiply the atomic mass of each element by the number of atoms of that element in the compound. For capsaicin, this would be (18 atoms C * 12.01 g/mol C) + (27 atoms H * 1.01 g/mol H) + (1 atom N * 14.01 g/mol N) + (3 atoms O * 16.00 g/mol O).
Step 3: Add up all the values obtained in step 2 to get the molar mass of capsaicin.
Step 4: To find the percentage by mass of carbon in capsaicin, divide the total mass of carbon in one mole of capsaicin by the molar mass of capsaicin. This would be (18 atoms C * 12.01 g/mol C) / molar mass of capsaicin.
Step 5: Multiply the result from step 4 by 100% to get the percentage by mass of carbon in capsaicin.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Molar Mass Calculation
Molar mass is the mass of one mole of a substance, typically expressed in grams per mole (g/mol). To calculate the molar mass of a compound, sum the molar masses of all the atoms in its chemical formula. For capsaicin (C18H27NO3), this involves calculating the contributions from carbon, hydrogen, nitrogen, and oxygen based on their respective atomic masses.
Recommended video:
Guided course
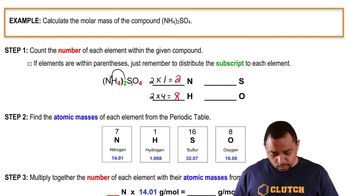
Molar Mass Calculation Example
Percentage by Mass
Percentage by mass is a way to express the concentration of an element within a compound. It is calculated by dividing the total mass of the element in the compound by the molar mass of the compound, then multiplying by 100. This provides a clear understanding of how much of the compound's mass is due to a specific element, such as carbon in capsaicin.
Recommended video:
Guided course

Mass Percent Calculation
Empirical and Molecular Formulas
The empirical formula represents the simplest whole-number ratio of elements in a compound, while the molecular formula shows the actual number of each type of atom in a molecule. Capsaicin's molecular formula (C18H27NO3) indicates the exact number of carbon, hydrogen, nitrogen, and oxygen atoms, which is essential for calculating the mass percentage of carbon accurately.
Recommended video:
Guided course
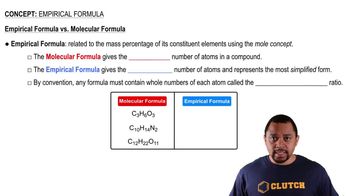
Empirical vs Molecular Formula
Related Practice
Textbook Question
Textbook Question
Calculate the percentage by mass of the indicated element in the following compounds: d. platinum in PtCl2(NH3)2, a chemotherapy agent called cisplatin
Textbook Question
Calculate the percentage by mass of the indicated element in the following compounds: e. oxygen in the female sex hormone estradiol, C18H24O2
Textbook Question
Based on the following structural formulas, calculate the percentage of carbon by mass present in each compound: (a) Benzaldehyde (almond fragrance) (b) Vanillin (vanilla flavor) c) Isopentyl acetate (banana flavor)
Textbook Question
Calculate the percentage of carbon by mass in each of the compounds represented by the following models: (a)
Textbook Question
(a) Write 'true' or 'false' for each statement. (a) A mole of horses contain a mole of horse legs.
2
views
