Balance the following equations: b. WCl6(𝑠)+Na2S(𝑠)⟶WS2(𝑠)+NaCl(𝑠)+S(𝑠)

Write balanced chemical equations corresponding to each of the following descriptions: c. Solid zinc metal reacts with sulfuric acid to form hydrogen gas and an aqueous solution of zinc sulfate.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Balancing Chemical Equations

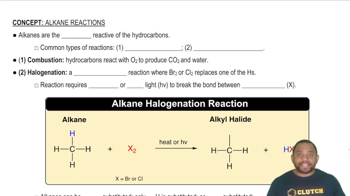
Types of Chemical Reactions
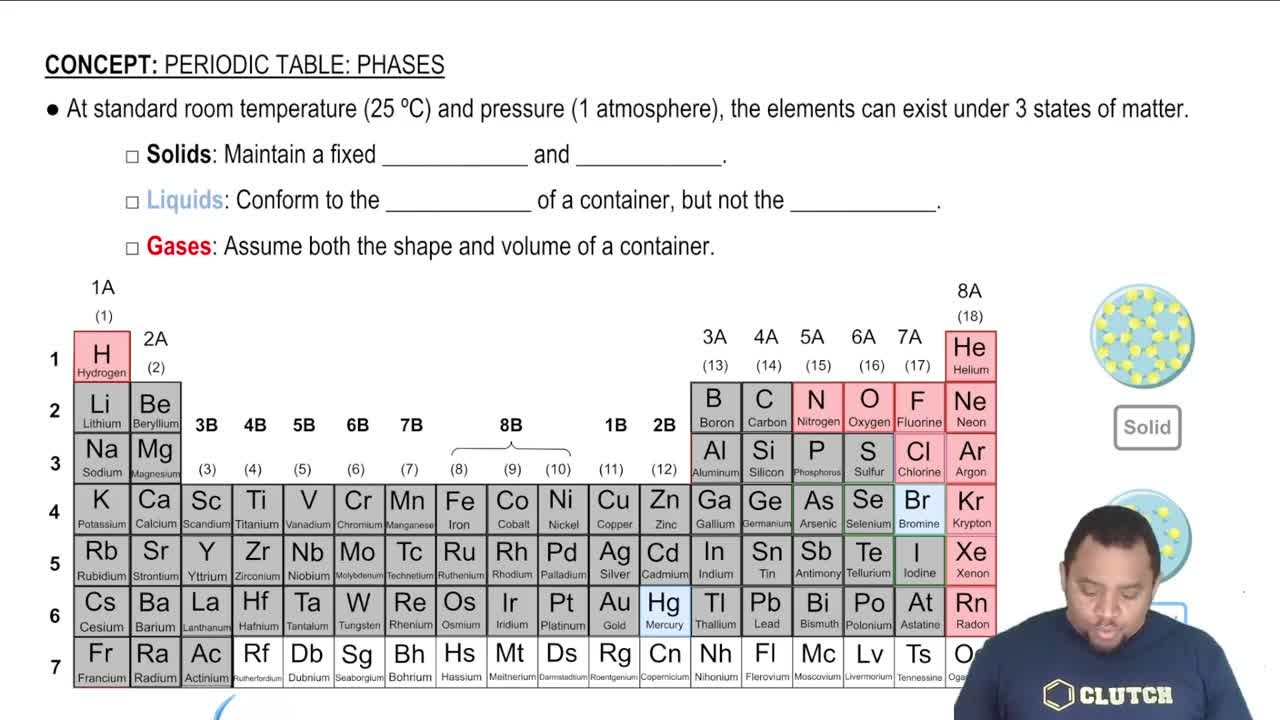
Chemical Formulas and States of Matter
Balance the following equations: c. NaHCO3(𝑠)+H2SO4(𝑎𝑞)⟶CO2(𝑔)+H2O(𝑙)+Na2SO4(𝑎𝑞)
Balance the following equations: d, NaN3(𝑠)+HNO2(𝑎𝑞)⟶N2(𝑔)+NO(𝑔)+NaOH(𝑎𝑞)
Write balanced chemical equations to correspond to each of the following descriptions: (a) When sulfur trioxide gas reacts with water, a solution of sulfuric acid forms. (b) Boron sulfide, B2S3(s), reacts violently with water to form dissolved boric acid, H3BO3, and hydrogen sulfide gas.
Write balanced chemical equations to correspond to each of the following descriptions: (c) Phosphine, PH3(g), combusts in oxygen gas to form water vapor and solid tetraphosphorus decaoxide.
Write balanced chemical equations to correspond to each of the following descriptions: (d) When solid mercury(II) nitrate is heated, it decomposes to form solid mercury(II) oxide, gaseous nitrogen dioxide, and oxygen. (e) Copper metal reacts with hot concentrated sulfuric acid solution to form aqueous copper(II) sulfate, sulfur dioxide gas, and water.
