Ch.23 - Transition Metals and Coordination Chemistry

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 23, Problem 29
Complete the exercises below. Polydentate ligands can vary in the number of coordination positions they occupy. In each of the following, identify the polydentate ligand present and indicate the probable number of coordination positions it occupies: a. [Co(NH₃)₄ (o-phen)] Cl₃.
 Verified step by step guidance
Verified step by step guidance1
Identify the ligands present in the complex. In [Co(NH₃)_4(o-phen)]Cl₃, the ligands are NH₃ (ammonia) and o-phen (ortho-phenanthroline).
Determine which ligand is polydentate. NH₃ is a monodentate ligand, meaning it can only occupy one coordination position. o-phen, however, is a polydentate ligand.
Understand the structure of o-phen (ortho-phenanthroline). It is a bidentate ligand, meaning it can form two bonds with the central metal ion, Co in this case.
Count the coordination positions occupied by o-phen. Since o-phen is bidentate, it occupies two coordination positions.
Summarize the findings: In the complex [Co(NH₃)_4(o-phen)]Cl₃, the polydentate ligand is o-phen, and it occupies two coordination positions.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Polydentate Ligands
Polydentate ligands are molecules that can attach to a central metal atom at multiple coordination sites. This ability allows them to form stable complexes with metal ions, as they create multiple bonds, enhancing the overall stability of the complex. Common examples include ethylenediamine and oxalate, which can bind through two or more donor atoms.
Recommended video:
Guided course
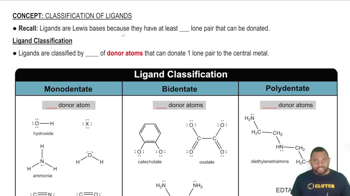
Ligand Classification
Coordination Number
The coordination number refers to the total number of ligand donor atoms that are bonded to a central metal ion in a coordination complex. It is determined by the number of bonds formed between the ligands and the metal. For polydentate ligands, the coordination number can be higher than for monodentate ligands, as they can occupy multiple positions simultaneously.
Recommended video:
Guided course

Coordination Numbers
Chelation
Chelation is the process by which a polydentate ligand forms a complex with a metal ion, effectively 'grabbing' the metal through multiple coordination sites. This interaction often results in more stable complexes compared to those formed with monodentate ligands. Chelating agents are widely used in various applications, including medicine and environmental science, to sequester metal ions.
Recommended video:
Guided course
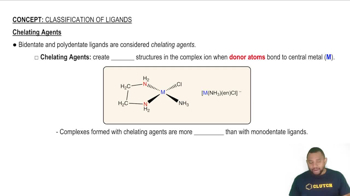
Chelating Agents
Related Practice
