Ch.23 - Transition Metals and Coordination Chemistry

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 23, Problem 43
Complete the exercises below. Determine if each of the following complexes exhibits geometric isomerism. If geometric isomers exist, determine how many there are. c. octahedral [Fe(o-phen)₂ Cl₂]⁺.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand the structure of the complex. The complex [Fe(o-phen)₂Cl₂]⁺ is an octahedral complex where 'o-phen' stands for 1,10-phenanthroline, a bidentate ligand, and there are two chloride ions as ligands.
Step 2: Identify the coordination number and geometry. The coordination number for this complex is 6, which is typical for octahedral complexes. The ligands are arranged around the central metal ion, Fe, in an octahedral geometry.
Step 3: Consider the arrangement of ligands. In an octahedral complex, geometric isomerism can occur if there are different types of ligands. Here, we have two bidentate o-phen ligands and two monodentate Cl⁻ ligands.
Step 4: Determine possible isomers. For octahedral complexes with the formula [M(A₂B₂)], where A and B are different ligands, geometric isomers can exist as 'cis' (adjacent) and 'trans' (opposite) forms. Apply this to [Fe(o-phen)₂Cl₂]⁺.
Step 5: Count the isomers. In this case, the complex can have two geometric isomers: one where the two Cl⁻ ligands are adjacent (cis) and one where they are opposite each other (trans).
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Geometric Isomerism
Geometric isomerism occurs when compounds have the same molecular formula and connectivity of atoms but differ in the spatial arrangement of those atoms. This type of isomerism is particularly relevant in coordination complexes, where ligands can occupy different positions around a central metal ion, leading to distinct isomers with different properties.
Recommended video:
Guided course
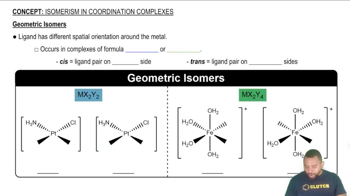
Geometric Isomers
Octahedral Coordination Complexes
Octahedral coordination complexes consist of a central metal ion surrounded by six ligands arranged at the corners of an octahedron. The geometry allows for various ligand arrangements, which can lead to the formation of geometric isomers, such as cis and trans isomers, depending on the relative positions of the ligands.
Recommended video:
Guided course
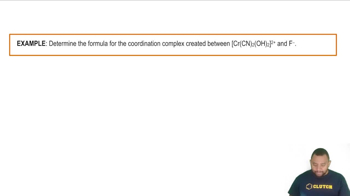
Coordination Complexes Example
Ligand Types and Their Influence
The type of ligands attached to a metal center can significantly influence the possibility of geometric isomerism. Bidentate ligands, like o-phenanthroline (o-phen), can create more complex arrangements compared to monodentate ligands, affecting the number and types of isomers that can form in octahedral complexes.
Recommended video:
Guided course
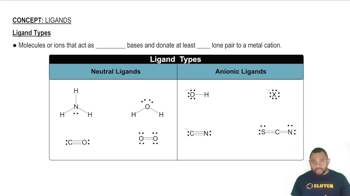
Ligand Types
Related Practice
