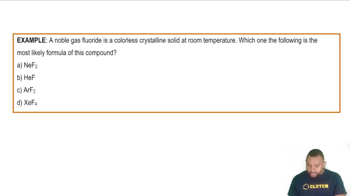
Halogens and Noble Gases
Halogens are a group of elements in Group 17 of the periodic table, known for their high reactivity and tendency to gain electrons, often resulting in negative oxidation states. Noble gases, found in Group 18, are generally inert and have a stable electron configuration, typically exhibiting a zero oxidation state. Understanding the behavior of these elements is crucial for predicting their chemical properties and reactions.

 Verified step by step guidance
Verified step by step guidance