Ch.22 - Chemistry of the Nonmetals

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 22, Problem 42
Complete the exercises below. Write the chemical formula for each of the following compounds, and indicate the oxidation state of the group 6A element in each: a. sulfur tetrachloride, b. selenium trioxide, c. sodium thiosulfate, d. hydrogen sulfide, e. sulfuric acid, f. sulfur dioxide, g. mercury telluride.
 Verified step by step guidance
Verified step by step guidance1
Identify the chemical formula for sulfur tetrachloride: Sulfur is S and tetrachloride indicates four chlorine atoms, so the formula is \( \text{SCl}_4 \). Determine the oxidation state of sulfur by considering that chlorine typically has an oxidation state of -1.
Determine the chemical formula for selenium trioxide: Selenium is Se and trioxide indicates three oxygen atoms, so the formula is \( \text{SeO}_3 \). Oxygen typically has an oxidation state of -2, use this to find the oxidation state of selenium.
Write the chemical formula for sodium thiosulfate: Sodium is Na, thiosulfate is a polyatomic ion \( \text{S}_2\text{O}_3^{2-} \), so the formula is \( \text{Na}_2\text{S}_2\text{O}_3 \). Determine the oxidation state of sulfur in the thiosulfate ion.
Identify the chemical formula for hydrogen sulfide: Hydrogen is H and sulfide indicates sulfur, so the formula is \( \text{H}_2\text{S} \). Determine the oxidation state of sulfur considering hydrogen typically has an oxidation state of +1.
Write the chemical formula for sulfuric acid: Sulfuric acid is \( \text{H}_2\text{SO}_4 \). Use the typical oxidation state of oxygen (-2) and hydrogen (+1) to find the oxidation state of sulfur.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Chemical Formulas
A chemical formula represents the composition of a compound, indicating the types and numbers of atoms present. For example, in sulfur tetrachloride (SCl4), the formula shows one sulfur atom bonded to four chlorine atoms. Understanding how to derive these formulas involves knowledge of valence electrons and bonding patterns.
Recommended video:
Guided course

Skeletal Formula
Oxidation States
The oxidation state, or oxidation number, of an element in a compound reflects its degree of oxidation or reduction. It is a useful concept for understanding electron transfer in reactions. For instance, in sulfuric acid (H2SO4), sulfur has an oxidation state of +6, indicating it has lost electrons compared to its elemental form.
Recommended video:
Guided course

Oxidation Numbers
Group 6A Elements
Group 6A elements, also known as the chalcogens, include oxygen, sulfur, selenium, and tellurium. These elements typically exhibit a range of oxidation states, commonly -2, 0, +4, and +6, depending on their bonding environment. Recognizing the typical oxidation states of these elements is crucial for determining their behavior in various compounds.
Recommended video:
Guided course
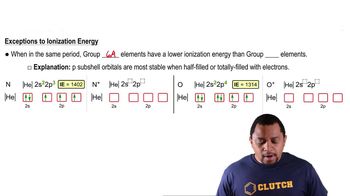
Group 6A vs. Group 5A Elements
Related Practice
