Ch.22 - Chemistry of the Nonmetals

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 22, Problem 61
Complete the exercises below. Write a balanced equation for each of the following reactions: a. preparation of white phosphorus from calcium phosphate, b. hydrolysis of PBr₃, c. reduction of PBr₃ to P₄ in the gas phase, using H₂.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Identify the reactants and products for each reaction. For reaction (a), the reactants are calcium phosphate and the products include white phosphorus (P₄). For reaction (b), the reactant is phosphorus tribromide (PBr₃) and the products include phosphorous acid (H₃PO₃) and hydrogen bromide (HBr). For reaction (c), the reactants are phosphorus tribromide (PBr₃) and hydrogen gas (H₂), and the product is white phosphorus (P₄).
Step 2: Write the unbalanced chemical equations for each reaction. For (a), start with Ca₃(PO₄)₂ → P₄. For (b), start with PBr₃ + H₂O → H₃PO₃ + HBr. For (c), start with PBr₃ + H₂ → P₄ + HBr.
Step 3: Balance the chemical equation for reaction (a). Balance the number of phosphorus atoms first, then balance calcium and oxygen atoms. Ensure that the number of atoms of each element is equal on both sides of the equation.
Step 4: Balance the chemical equation for reaction (b). Start by balancing the phosphorus atoms, then balance the bromine atoms, and finally balance the hydrogen and oxygen atoms. Make sure the number of atoms for each element is the same on both sides.
Step 5: Balance the chemical equation for reaction (c). Begin by balancing the phosphorus atoms, then balance the bromine atoms, and finally balance the hydrogen atoms. Check that the number of atoms for each element is equal on both sides of the equation.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Balancing Chemical Equations
Balancing chemical equations involves ensuring that the number of atoms for each element is the same on both the reactant and product sides. This is based on the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. To balance an equation, coefficients are adjusted in front of the chemical formulas.
Recommended video:
Guided course

Balancing Chemical Equations
Types of Chemical Reactions
Understanding the types of chemical reactions is crucial for writing balanced equations. Common types include synthesis, decomposition, single replacement, and double replacement reactions. Each type has specific characteristics that dictate how reactants interact and what products are formed, guiding the formulation of the balanced equation.
Recommended video:
Guided course
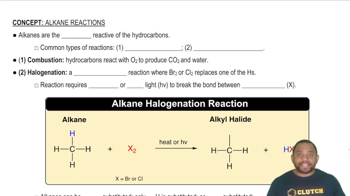
Common Types of Alkane Reactions
Stoichiometry
Stoichiometry is the quantitative relationship between reactants and products in a chemical reaction. It allows chemists to predict the amounts of substances consumed and produced in a reaction based on balanced equations. Mastery of stoichiometry is essential for accurately writing and interpreting balanced equations, especially in complex reactions.
Recommended video:
Guided course

Stoichiometry Concept
Related Practice
