Ch.22 - Chemistry of the Nonmetals

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 22, Problem 16
Complete the exercises below. Which of the following statements are true? a. Si can form an ion with six fluorine atoms, SiF₆²⁻, whereas carbon cannot. b. Si can form three stable compounds containing two Si atoms each, Si₂H₂, Si₂H₄, and Si₂H₆. c. In HNO₃ and H₃PO₄, the central atoms, N and P, have different oxidation states. d. S is more electronegative than Se.
 Verified step by step guidance
Verified step by step guidance1
Step 1: Analyze statement (a). Consider the ability of silicon (Si) and carbon (C) to form ions with fluorine. Silicon can expand its octet due to available d-orbitals, allowing it to form SiF₆²⁻, while carbon cannot expand its octet beyond four bonds.
Step 2: Evaluate statement (b). Investigate the existence of stable silicon compounds with two silicon atoms. Check known silicon hydrides and their stability to determine if Si₂H₂, Si₂H₄, and Si₂H₆ are stable compounds.
Step 3: Examine statement (c). Determine the oxidation states of nitrogen (N) in HNO₃ and phosphorus (P) in H₃PO₄. Use the rules for assigning oxidation states to calculate and compare the oxidation states of N and P in these compounds.
Step 4: Assess statement (d). Compare the electronegativity values of sulfur (S) and selenium (Se) using the Pauling scale. Electronegativity generally decreases down a group in the periodic table, so verify if S is indeed more electronegative than Se.
Step 5: Summarize the findings for each statement, identifying which are true based on the analysis of chemical bonding, compound stability, oxidation states, and electronegativity.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Ionic Compounds and Coordination
Ionic compounds form when atoms transfer electrons, resulting in charged ions. Silicon (Si) can form a hexafluorosilicate ion, SiF₆²⁻, by coordinating with six fluoride ions, which is a characteristic of its ability to expand its coordination number. In contrast, carbon typically forms covalent bonds and does not have the same capacity for coordination with multiple anions.
Recommended video:
Guided course
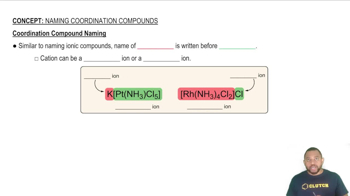
Coordination Compound Naming
Silicon Compounds and Allotropes
Silicon can form various stable compounds, including silanes like Si₂H₂, Si₂H₄, and Si₂H₆, which are characterized by different hydrogen saturation levels. These compounds illustrate silicon's ability to form multiple bonds and its versatility in bonding, which is distinct from carbon's bonding behavior in similar compounds.
Recommended video:
Guided course

Ionic Compounds Naming
Oxidation States and Electronegativity
Oxidation states indicate the degree of oxidation of an atom in a compound, reflecting the number of electrons lost or gained. In HNO₃ and H₃PO₄, nitrogen (N) and phosphorus (P) exhibit different oxidation states due to their differing positions in the periodic table. Additionally, electronegativity is a measure of an atom's ability to attract electrons; sulfur (S) is more electronegative than selenium (Se), influencing their chemical behavior and reactivity.
Recommended video:
Guided course

Electronegativity Trends
Related Practice
