Ch.22 - Chemistry of the Nonmetals

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 22, Problem 23
Complete the exercises below. Complete and balance the following equation: b. Fe (s) + H₂SO₄ (aq) →
 Verified step by step guidance
Verified step by step guidance1
Identify the reactants and products in the chemical equation. Here, the reactants are Fe (s) and H₂SO₄ (aq), and the products will include FeSO₄ (aq) and H₂ (g).
Write the unbalanced chemical equation: Fe (s) + H₂SO₄ (aq) → FeSO₄ (aq) + H₂ (g).
Count the number of each type of atom on both sides of the equation to determine which elements need balancing.
Balance the equation by adjusting coefficients. Start by balancing the number of Fe atoms, then balance the number of H atoms, and finally the number of S and O atoms.
Verify that the equation is balanced by ensuring that the number of each type of atom is the same on both sides of the equation.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Balancing Chemical Equations
Balancing chemical equations involves ensuring that the number of atoms of each element is the same on both sides of the equation. This is based on the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. To balance an equation, coefficients are adjusted in front of the chemical formulas to achieve equal atom counts.
Recommended video:
Guided course

Balancing Chemical Equations
Types of Chemical Reactions
The reaction between iron (Fe) and sulfuric acid (H₂SO₄) is an example of a single displacement reaction, where one element displaces another in a compound. In this case, iron displaces hydrogen from sulfuric acid, forming iron(II) sulfate and hydrogen gas. Understanding the type of reaction helps predict the products formed and the balancing process.
Recommended video:
Guided course
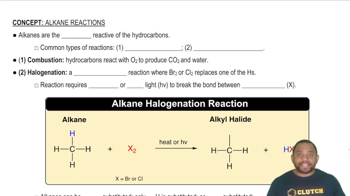
Common Types of Alkane Reactions
Stoichiometry
Stoichiometry is the calculation of reactants and products in chemical reactions based on balanced equations. It allows chemists to determine the amounts of substances consumed and produced in a reaction. In the context of the given equation, stoichiometry will help in calculating the moles of iron and sulfuric acid needed to fully react and produce the expected products.
Recommended video:
Guided course

Stoichiometry Concept
Related Practice
