Ch.22 - Chemistry of the Nonmetals

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 22, Problem 53
Complete the exercises below. Complete and balance the following equations: a. Mg₃N₂ (s) + H₂O (l) → Which ones of these are redox reactions?
 Verified step by step guidance
Verified step by step guidance1
Step 1: Write the unbalanced chemical equation for the reaction: \( \text{Mg}_3\text{N}_2 (s) + \text{H}_2\text{O} (l) \rightarrow \text{?} \).
Step 2: Determine the products of the reaction. Magnesium nitride reacts with water to form magnesium hydroxide and ammonia: \( \text{Mg}_3\text{N}_2 + 6\text{H}_2\text{O} \rightarrow 3\text{Mg(OH)}_2 + 2\text{NH}_3 \).
Step 3: Balance the chemical equation by ensuring the number of atoms of each element is the same on both sides of the equation. Start by balancing magnesium, then nitrogen, and finally hydrogen and oxygen.
Step 4: Check if the reaction is a redox reaction. A redox reaction involves the transfer of electrons between species. Identify the oxidation states of elements in the reactants and products to see if there is a change.
Step 5: Conclude whether the reaction is a redox reaction based on the changes in oxidation states. If there is no change in oxidation states, it is not a redox reaction.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Balancing Chemical Equations
Balancing chemical equations involves ensuring that the number of atoms for each element is the same on both sides of the equation. This is based on the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. To balance an equation, coefficients are adjusted in front of the chemical formulas to achieve equal atom counts.
Recommended video:
Guided course

Balancing Chemical Equations
Redox Reactions
Redox reactions, or reduction-oxidation reactions, involve the transfer of electrons between substances, resulting in changes in oxidation states. In these reactions, one species is oxidized (loses electrons) while another is reduced (gains electrons). Identifying redox reactions requires analyzing the oxidation states of the elements involved before and after the reaction.
Recommended video:
Guided course
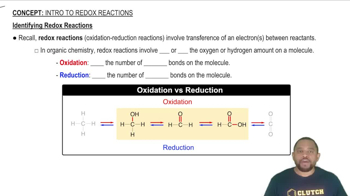
Identifying Redox Reactions
Oxidation States
Oxidation states (or oxidation numbers) are assigned to atoms in a compound to indicate their degree of oxidation or reduction. These states help in tracking electron transfer during chemical reactions. Common rules for assigning oxidation states include that the oxidation state of an element in its elemental form is zero, and for monoatomic ions, it equals the charge of the ion.
Recommended video:
Guided course

Oxidation Numbers
Related Practice
