Ch.22 - Chemistry of the Nonmetals

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 22, Problem 18
Complete the exercises below. Complete and balance the following equation: a. Mg₃N₂ (s) + H₂O (l) →
 Verified step by step guidance
Verified step by step guidance1
Identify the reactants and products in the reaction. The reactants are magnesium nitride (Mg₃N₂) and water (H₂O). The products will be magnesium hydroxide (Mg(OH)₂) and ammonia (NH₃).
Write the unbalanced chemical equation: Mg₃N₂ + H₂O → Mg(OH)₂ + NH₃.
Balance the magnesium atoms. There are 3 magnesium atoms in Mg₃N₂, so you need 3 Mg(OH)₂ on the product side: Mg₃N₂ + H₂O → 3 Mg(OH)₂ + NH₃.
Balance the nitrogen atoms. There are 2 nitrogen atoms in Mg₃N₂, so you need 2 NH₃ on the product side: Mg₃N₂ + H₂O → 3 Mg(OH)₂ + 2 NH₃.
Balance the hydrogen and oxygen atoms. Count the total number of hydrogen and oxygen atoms on both sides and adjust the coefficient of H₂O to balance the equation.
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Balancing Chemical Equations
Balancing chemical equations involves ensuring that the number of atoms of each element is the same on both sides of the equation. This is based on the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. To balance an equation, coefficients are adjusted in front of the chemical formulas to achieve equal atom counts.
Recommended video:
Guided course

Balancing Chemical Equations
Types of Chemical Reactions
Understanding the types of chemical reactions is crucial for predicting products. The reaction between magnesium nitride (Mg₃N₂) and water (H₂O) is a hydrolysis reaction, where a metal nitride reacts with water to produce hydroxides and ammonia. Recognizing the type of reaction helps in determining the expected products and their states.
Recommended video:
Guided course
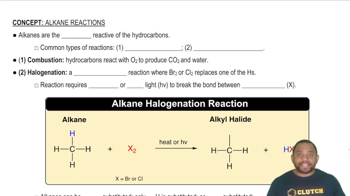
Common Types of Alkane Reactions
States of Matter in Chemical Reactions
In chemical equations, the states of matter (solid, liquid, gas, aqueous) are indicated to provide context for the reaction conditions. For example, Mg₃N₂ is a solid (s) and H₂O is a liquid (l). Knowing the states helps in understanding the physical behavior of reactants and products, which can influence reaction rates and mechanisms.
Recommended video:
Guided course
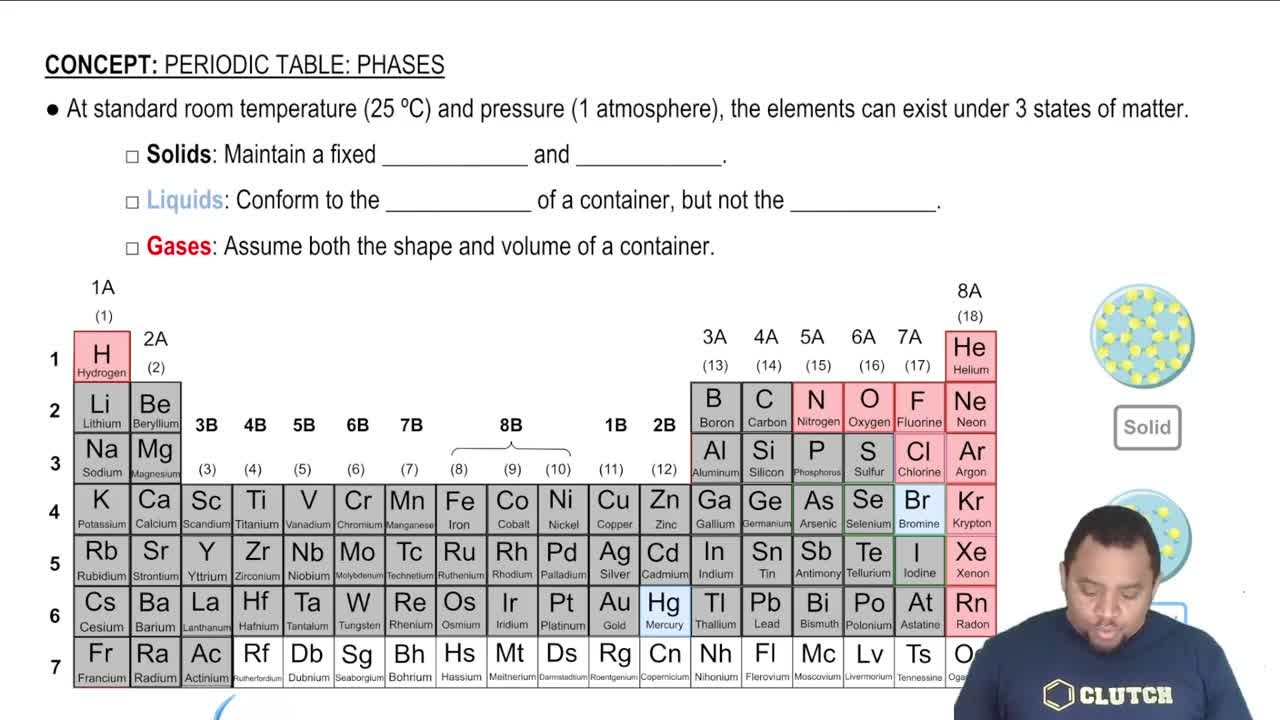
Element States of Matter
Related Practice
