Textbook Question
Decay of which nucleus will lead to the following products: (a) bismuth-211 by beta decay?

 Verified step by step guidance
Verified step by step guidance
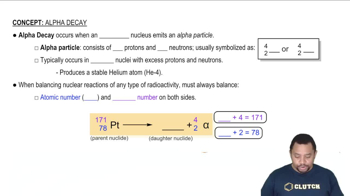
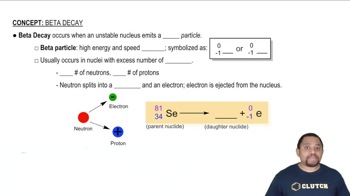
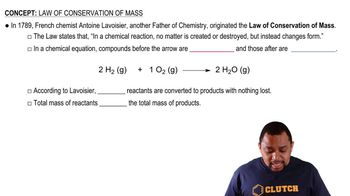
Decay of which nucleus will lead to the following products: (a) bismuth-211 by beta decay?
Decay of which nucleus will lead to the following products: (b) chromium-50 by positron emission?
Decay of which nucleus will lead to the following products: (c) tantalum-179 by electron capture?
Predict the type of radioactive decay process for the following radionuclides: (a) 85B.