In 2010, a team of scientists from Russia and the United States reported creation of the first atom of element 117, which is named tennessine, and whose symbol is Ts. The synthesis involved the collision of a target of 24997Bk with accelerated ions of an isotope which we will denote Q. The product atom, which we will call Z, immediately releases neutrons and forms 294117Ts: 24997Bk + Q → Z → 294117Ts + 3 10n (a) What are the identities of isotopes Q and Z? (c) Collision of ions of isotope Q with a target was also used to produce the first atoms of livermorium, Lv. The initial product of this collision was 296116Lv. What was the target isotope with which Q collided in this experiment?

Each of the following transmutations produces a radionuclide used in positron emission tomography (PET).
(a) In equations (i) and (ii), identify the species signified as 'X.'
(i) 14N(p,α)X
(ii) 18O(p,X)18F
(iii) 14N(d,n)15O
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Nuclear Transmutation
Radionuclides and Positron Emission Tomography (PET)
Nuclear Notation

In 2010, a team of scientists from Russia and the United States reported creation of the first atom of element 117, which is named tennessine, and whose symbol is Ts. The synthesis involved the collision of a target of 24997Bk with accelerated ions of an isotope which we will denote Q. The product atom, which we will call Z, immediately releases neutrons and forms 294117Ts: 24997Bk + Q → Z → 294117Ts + 3 10n (b) Isotope Q is unusual in that it is very long-lived (its half-life is on the order of 1019 yr) in spite of having an unfavorable neutron-to-proton ratio (Figure 21.1). Can you propose a reason for its unusual stability?
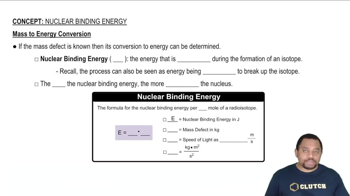
The nuclear masses of 7Be, 9Be, and 10Be are 7.0147, 9.0100, and 10.0113 amu, respectively. Which of these nuclei has the largest binding energy per nucleon?
