The table provided gives the number of protons (p) and neutrons (n) for four isotopes, identified only as (i)–(iv). a. Write the symbol for each of the isotopes.

Chlorine has two stable nuclides, 35Cl and 37Cl. In contrast, 36Cl is a radioactive nuclide that decays by beta emission. (a) What is the product of decay of 36Cl?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Beta Decay

Nuclear Stability
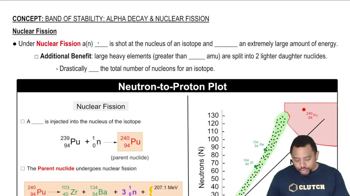
Decay Products

Radon-222 decays to a stable nucleus by a series of three alpha emissions and two beta emissions. What is the stable nucleus that is formed?
Chlorine has two stable nuclides, 35Cl and 37Cl. In contrast, 36Cl is a radioactive nuclide that decays by beta emission. (b) Based on the empirical rules about nuclear stability, explain why the nucleus of 36Cl is less stable than either 35Cl or 37Cl.
Nuclear scientists have synthesized approximately 1600 nuclei not known in nature. More might be discovered with heavy-ion bombardment using high-energy particle accelerators. Complete and balance the following reactions, which involve heavy-ion bombardments:
(a) 63Li + 5628Ni → ?
(b) 4020Ca + 24896Cm → 14762Sm + ?
(c) 8838Sr + 8436Kr → 11646Pd + ?
(d) 4020Ca + 23892U → 7030Zn + 4 10n + 2 ?
In 2010, a team of scientists from Russia and the United States reported creation of the first atom of element 117, which is named tennessine, and whose symbol is Ts. The synthesis involved the collision of a target of 24997Bk with accelerated ions of an isotope which we will denote Q. The product atom, which we will call Z, immediately releases neutrons and forms 294117Ts: 24997Bk + Q → Z → 294117Ts + 3 10n (a) What are the identities of isotopes Q and Z? (c) Collision of ions of isotope Q with a target was also used to produce the first atoms of livermorium, Lv. The initial product of this collision was 296116Lv. What was the target isotope with which Q collided in this experiment?
