A voltaic cell that uses the reaction PdCl42-(aq) + Cd(s) → Pd(s) + 4 Cl-(aq) + Cd2+(aq) has a measured standard cell potential of +1.03 V. (b) By using data from Appendix E, determine E°red for the reaction involving Pd.
Ch.20 - Electrochemistry

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 20, Problem 41b
Using standard reduction potentials (Appendix E), calculate the standard emf for each of the following reactions: (b) Ni1s2 + 2 Ce4+1aq2 ¡ Ni2+1aq2 + 2 Ce3+1aq2
 Verified step by step guidance
Verified step by step guidance1
Identify the half-reactions involved in the overall redox reaction. For the given reaction, the half-reactions are: \( \text{Ni} \rightarrow \text{Ni}^{2+} + 2e^- \) and \( \text{Ce}^{4+} + e^- \rightarrow \text{Ce}^{3+} \).
Look up the standard reduction potentials for each half-reaction in Appendix E. The standard reduction potential for \( \text{Ni}^{2+} + 2e^- \rightarrow \text{Ni} \) is \( E^\circ = -0.25 \text{ V} \), and for \( \text{Ce}^{4+} + e^- \rightarrow \text{Ce}^{3+} \) is \( E^\circ = +1.61 \text{ V} \).
Reverse the reduction potential for the oxidation half-reaction. Since \( \text{Ni} \rightarrow \text{Ni}^{2+} + 2e^- \) is the oxidation half-reaction, its potential becomes \( E^\circ = +0.25 \text{ V} \).
Calculate the standard emf (electromotive force) of the cell by adding the standard potentials of the oxidation and reduction half-reactions: \( E^\circ_{\text{cell}} = E^\circ_{\text{reduction}} + E^\circ_{\text{oxidation}} \).
Substitute the values into the equation: \( E^\circ_{\text{cell}} = 1.61 \text{ V} + 0.25 \text{ V} \).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
4mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Standard Reduction Potentials
Standard reduction potentials are measured voltages that indicate the tendency of a chemical species to gain electrons and be reduced. Each half-reaction has a specific potential, typically measured under standard conditions (1 M concentration, 1 atm pressure, and 25°C). These values are crucial for predicting the direction of redox reactions and calculating the overall cell potential.
Recommended video:
Guided course

Standard Reduction Potentials
Electrochemical Cell and EMF
An electrochemical cell consists of two half-cells where oxidation and reduction reactions occur. The electromotive force (EMF) of the cell is the voltage generated by the spontaneous redox reaction, calculated as the difference between the reduction potentials of the cathode and anode. A positive EMF indicates a spontaneous reaction, while a negative EMF suggests non-spontaneity.
Recommended video:
Guided course
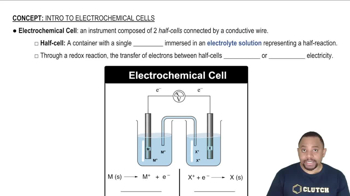
Electrochemical Cells
Balancing Redox Reactions
Balancing redox reactions involves ensuring that both mass and charge are conserved in the reaction. This is done by identifying oxidation and reduction half-reactions, balancing the number of electrons transferred, and adjusting coefficients accordingly. Properly balanced reactions are essential for accurate calculations of standard EMF and understanding the stoichiometry of the reaction.
Recommended video:
Guided course

Balancing Basic Redox Reactions
Related Practice
Textbook Question
Textbook Question
A voltaic cell that uses the reaction PdCl42-(aq) + Cd(s) → Pd(s) + 4 Cl-(aq) + Cd2+(aq) has a measured standard cell potential of +1.03 V. (c) Sketch the voltaic cell, label the anode and cathode, and indicate the direction of electron flow
Textbook Question
Using standard reduction potentials (Appendix E), calculate the standard emf for each of the following reactions: (a) Cl21g2 + 2 I-1aq2 ¡ 2 Cl-1aq2 + I21s2
Textbook Question
Using standard reduction potentials (Appendix E), calculate the standard emf for each of the following reactions: (c) Fe1s2 + 2 Fe3+1aq2 ¡ 3 Fe2+1aq2
Textbook Question
Using standard reduction potentials (Appendix E), calculate the standard emf for each of the following reactions: (d) 2 NO3-1aq2 + 8 H+1aq2 + 3 Cu1s2 ¡ 2 NO1g2 + 4 H2O1l2 + 3 Cu2+1aq2
