Textbook Question
The diagram that follows represents a molecular view of a process occurring at an electrode in a voltaic cell.
(a) Does the process represent oxidation or reduction?
3
views


 Verified step by step guidance
Verified step by step guidance


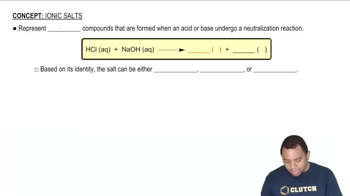
The diagram that follows represents a molecular view of a process occurring at an electrode in a voltaic cell.
(a) Does the process represent oxidation or reduction?
The diagram that follows represents a molecular view of a process occurring at an electrode in a voltaic cell.
(b) Is the electrode the anode or cathode?
The diagram that follows represents a molecular view of a process occurring at an electrode in a voltaic cell.
(c) Why are the atoms in the electrode represented by larger spheres than those in the solution? [Section 20.3]
Consider the following table of standard electrode potentials for a series of hypothetical reactions in an aqueous solution: reduction half-reaction E °(V) (c) Which substance(s) can oxidize C2+?