Cytochrome, a complicated molecule that we will represent as CyFe2+, reacts with the air we breathe to supply energy required to synthesize adenosine triphosphate (ATP). The body uses ATP as an energy source to drive other reactions (Section 19.7). At pH 7.0 the following reduction potentials pertain to this oxidation of CyFe2+: O21g2 + 4 H+1aq2 + 4 e- ¡ 2 H2O1l2 Ered ° = +0.82 V CyFe3+1aq2 + e- ¡ CyFe2+1aq2 E°red = +0.22 V (a) What is ∆G for the oxidation of CyFe2+ by air? (b) If the synthesis of 1.00 mol of ATP from adenosine diphosphate (ADP) requires a ∆G of 37.7 kJ, how many moles of ATP are synthesized per mole of O2?
Ch.20 - Electrochemistry

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 20, Problem 115
A student designs an ammeter (a device that measures electrical current) that is based on the electrolysis of water into hydrogen and oxygen gases. When electrical current of unknown magnitude is run through the device for 2.00 min, 12.3 mL of water-saturated H21g2 is collected. The temperature of the system is 25.5 °C, and the atmospheric pressure is 768 torr. What is the magnitude of the current in amperes?
 Verified step by step guidance
Verified step by step guidance1
Identify the chemical reaction: The electrolysis of water produces hydrogen gas (H₂) and oxygen gas (O₂). The balanced equation is 2H₂O(l) → 2H₂(g) + O₂(g).
Determine the moles of hydrogen gas produced: Use the ideal gas law, PV = nRT, to find the moles of H₂. First, convert the volume of H₂ from mL to L, the temperature from °C to Kelvin, and the pressure from torr to atm.
Account for water vapor pressure: Since the hydrogen gas is water-saturated, subtract the vapor pressure of water at 25.5 °C from the total pressure to find the pressure of the dry hydrogen gas.
Calculate the charge passed: Use the stoichiometry of the reaction. From the balanced equation, 2 moles of electrons are required to produce 1 mole of H₂. Use Faraday's constant (96485 C/mol) to find the total charge in coulombs.
Determine the current: Use the formula I = Q/t, where I is the current in amperes, Q is the charge in coulombs, and t is the time in seconds (convert 2.00 min to seconds).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
11mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Electrolysis of Water
Electrolysis of water is a chemical process that uses electrical energy to decompose water (H2O) into its constituent gases, hydrogen (H2) and oxygen (O2). This process occurs in an electrolytic cell where an electric current is passed through water containing an electrolyte, facilitating the separation of water molecules. The volume of gas produced can be measured to determine the amount of current used, as the amount of gas generated is directly proportional to the charge passed.
Recommended video:
Guided course
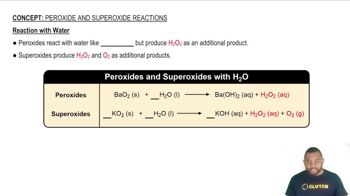
Reaction with Water
Ideal Gas Law
The Ideal Gas Law is a fundamental equation in chemistry that relates the pressure, volume, temperature, and number of moles of a gas. It is expressed as PV = nRT, where P is pressure, V is volume, n is the number of moles, R is the ideal gas constant, and T is temperature in Kelvin. This law allows for the calculation of gas quantities under varying conditions, which is essential for determining the amount of hydrogen gas produced during electrolysis in the given problem.
Recommended video:
Guided course

Ideal Gas Law Formula
Faraday's Laws of Electrolysis
Faraday's Laws of Electrolysis describe the relationship between the amount of substance produced at an electrode during electrolysis and the quantity of electric charge passed through the electrolyte. The first law states that the mass of a substance altered at an electrode is directly proportional to the total electric charge passed. This principle is crucial for calculating the current in the ammeter design, as it links the volume of hydrogen gas collected to the current flowing through the system over time.
Recommended video:
Guided course
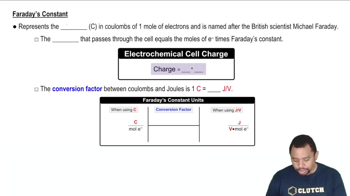
Faraday's Constant in Electrochemistry
Related Practice
Textbook Question
Textbook Question
Cytochrome, a complicated molecule that we will represent as CyFe2+, reacts with the air we breathe to supply energy required to synthesize adenosine triphosphate (ATP). The body uses ATP as an energy source to drive other reactions (Section 19.7). At pH 7.0 the following reduction potentials pertain to this oxidation of CyFe2+: O21g2 + 4 H+1aq2 + 4 e- ¡ 2 H2O1l2 Ered ° = +0.8 (b) If the synthesis of 1.00 mol of ATP from adenosine diphosphate (ADP) requires a ∆G of 37.7 kJ, how many moles of ATP are synthesized per mole of O2?
