Using standard reduction potentials (Appendix E), calculate the standard emf for each of the following reactions: (d) 2 NO3-1aq2 + 8 H+1aq2 + 3 Cu1s2 ¡ 2 NO1g2 + 4 H2O1l2 + 3 Cu2+1aq2

A 1 M solution of Cu(NO3)2 is placed in a beaker with a strip of Cu metal. A 1 M solution of SnSO4 is placed in a second beaker with a strip of Sn metal. A salt bridge connects the two beakers, and wires to a voltmeter link the two metal electrodes. (a) Which electrode serves as the anode, and which as the cathode? (b) Which electrode gains mass, and which loses mass as the cell reaction proceeds?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
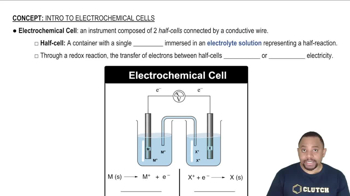
Electrochemical Cells
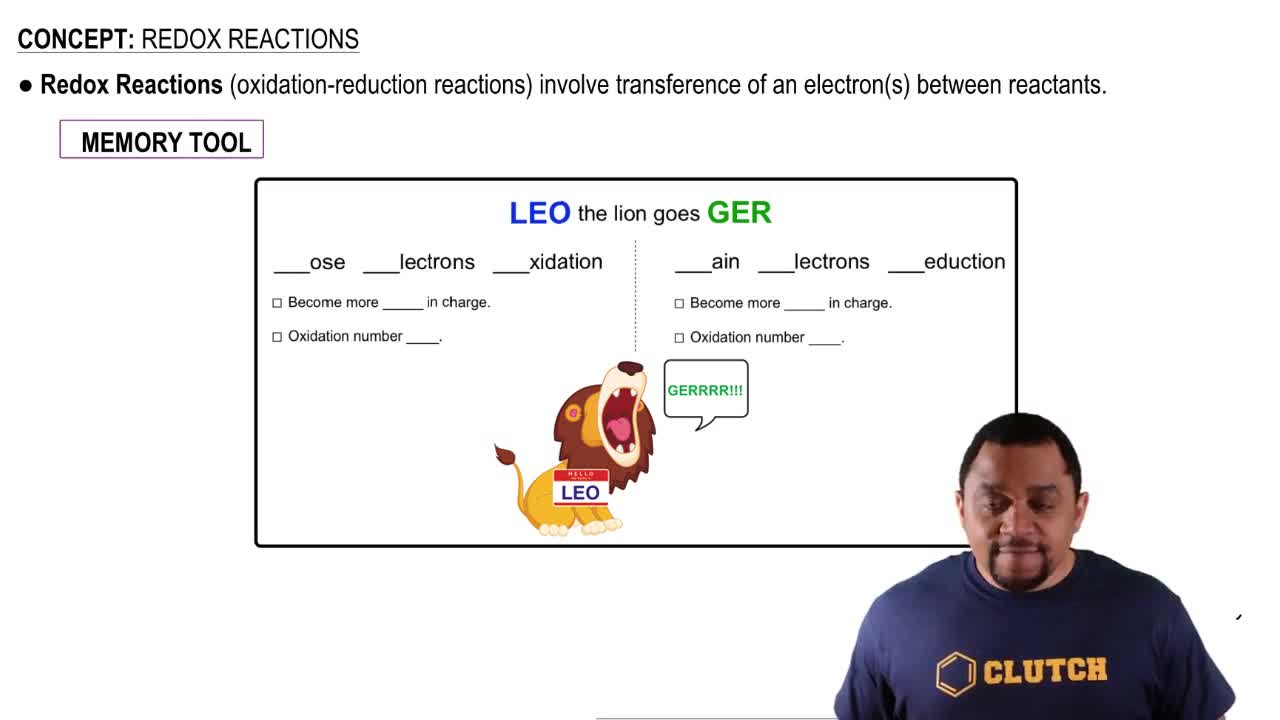
Oxidation and Reduction
Salt Bridge Function
The standard reduction potentials of the following halfreactions are given in Appendix E:
Ag+(aq) + e- → Ag(s)
Cu2+(aq) + 2 e- → Cu(s)
Ni2+(aq) + 2 e- → Ni(s)
Cr3+(aq) + 3 e- → Cr(s)
(a) Determine which combination of these half-cell reactions leads to the cell reaction with the largest positive cell potential and calculate the value.
(b) Determine which combination of these half-cell reactions leads to the cell reaction with the smallest positive cell potential and calculate the value.
A voltaic cell consists of a strip of cadmium metal in a solution of Cd(NO3)2 in one beaker, and in the other beaker a platinum electrode is immersed in a NaCl solution, with Cl2 gas bubbled around the electrode. A salt bridge connects the two beakers. (a) Which electrode serves as the anode, and which as the cathode? (b) Does the Cd electrode gain or lose mass as the cell reaction proceeds? (c) Write the equation for the overall cell reaction.
From each of the following pairs of substances, use data in Appendix E to choose the one that is the stronger reducing agent: (a) Fe(s) or Mg(s) (b) Ca(s) or Al(s) (c) H2(g, acidic solution) or H2S(g)
