Suppose a scientist repeats the Millikan oil-drop experiment but reports the charges on the drops using an unusual (and imaginary) unit called the warmomb (wa). The scientist obtains the following data for four of the drops: Droplet Calculated Charge (wa) A 3.84⨉10−8 B 4.80⨉10−8 C 2.88⨉10−8 D 8.64⨉10−8 (a) If all the droplets were the same size, which would fall most slowly through the apparatus?

Suppose a scientist repeats the Millikan oil-drop experiment but reports the charges on the drops using an unusual (and imaginary) unit called the warmomb (wa). The scientist obtains the following data for four of the drops: Droplet Calculated Charge (wa) A 3.84⨉10−8 B 4.80⨉10−8 C 2.88⨉10−8 D 8.64⨉10−8 (d) What is the conversion factor between warmombs and coulombs?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Millikan Oil-Drop Experiment
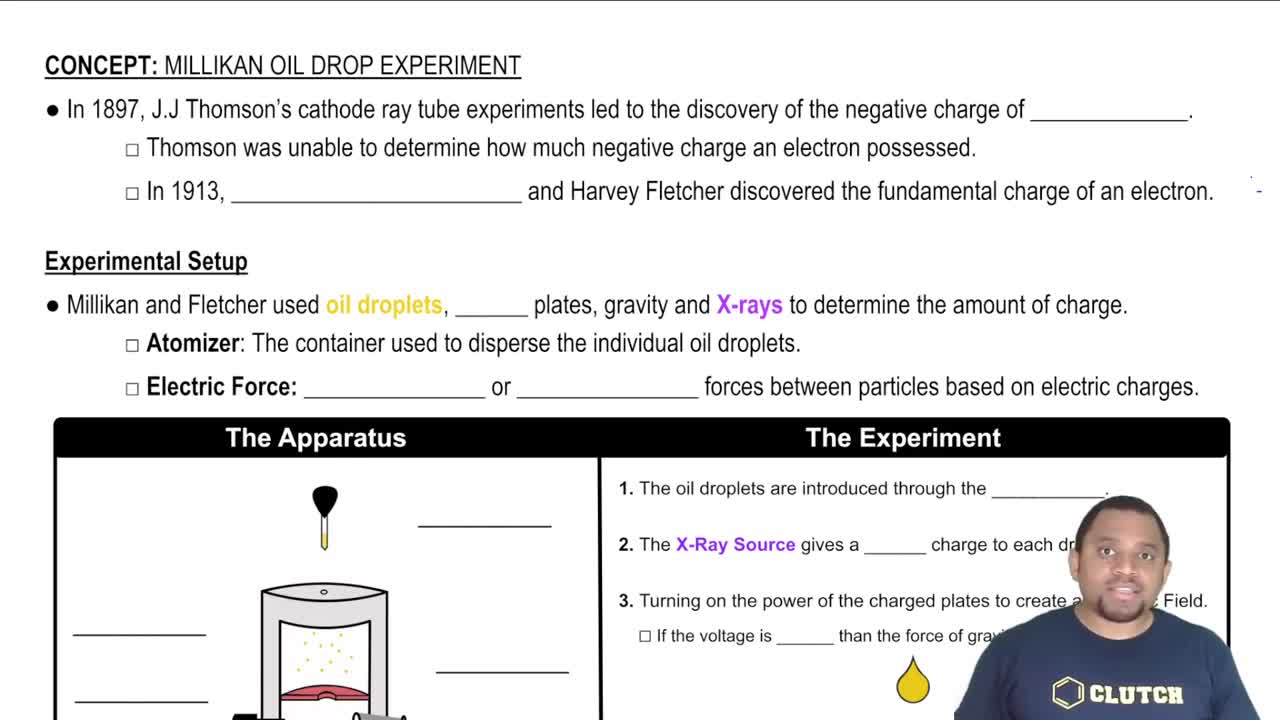
Unit Conversion

Quantization of Charge

Suppose a scientist repeats the Millikan oil-drop experiment but reports the charges on the drops using an unusual (and imaginary) unit called the warmomb (wa). The scientist obtains the following data for four of the drops: Droplet Calculated Charge (wa) A 3.84⨉10−8 B 4.80⨉10−8 C 2.88⨉10−8 D 8.64⨉10−8 (b) From these data, what is the best choice for the charge of the electron in warmombs?
Suppose a scientist repeats the Millikan oil-drop experiment but reports the charges on the drops using an unusual (and imaginary) unit called the warmomb (wa). The scientist obtains the following data for four of the drops: Droplet Calculated Charge (wa) A 3.84⨉10−8 B 4.80⨉10−8 C 2.88⨉10−8 D 8.64⨉10−8 (c) Based on your answer to part (b), how many electrons are there on each of the droplets?
The natural abundance of 3He is 0.000137%. (a) How many protons, neutrons, and electrons are in an atom of 3He?
The natural abundance of 3He is 0.000137%. (b) Based on the sum of the masses of their subatomic particles, which is expected to be more massive, an atom of 3He or an atom of 3H (which is also called tritium)?
The natural abundance of 3He is 0.000137%. (c) Based on your answer to part (b), what would need to be the precision of a mass spectrometer that is able to differentiate between peaks that are due to 3He+ and 3H+?
