Textbook Question
Name the following ionic compounds: (j) (NH4)2SO4.

 Verified step by step guidance
Verified step by step guidance


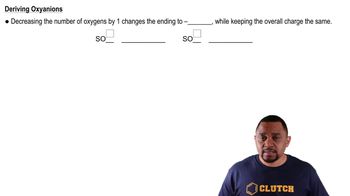
Name the following ionic compounds: (j) (NH4)2SO4.
Name the following ionic compounds: (a) KCN (b) NaBrO2 (c) Sr(OH)2 (d) CoTe
Name the following ionic compounds: (e) Fe2(CO3)3
Name the following ionic compounds: (g) (NH4)2SO3
Name the following ionic compounds: (h) NaH2PO4 (i) KMnO4
Name the following ionic compounds: (j) Ag2Cr2O7.