Textbook Question
How many of the indicated atoms are represented by each chemical formula: (b) oxygen atoms in Ca(ClO3)2 (c) hydrogen atoms in (NH4)2HPO4?

 Verified step by step guidance
Verified step by step guidance

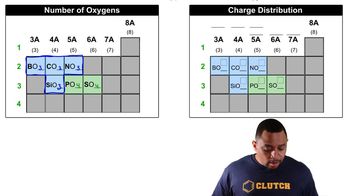

How many of the indicated atoms are represented by each chemical formula: (b) oxygen atoms in Ca(ClO3)2 (c) hydrogen atoms in (NH4)2HPO4?
Write the molecular and structural formulas for the compounds represented by the following molecular models:
Write the molecular and structural formulas for the compounds represented by the following models:
Fill in the gaps in the following table:
Symbol 31P3-
Protons 34 50
Neutrons 45 69 118
Electrons 46 76
Net charge 2- 3+
Each of the following elements is capable of forming an ion in chemical reactions. By referring to the periodic table, predict the charge of the most stable ion of each: c. K
Using the periodic table, predict the charge of the most stable ion of the following elements: d. Br