Elements in the same group of the periodic table often form oxyanions with the same general formula. The anions are also named in a similar fashion. Based on these observations, suggest a chemical formula or name, as appropriate, for each of the following ions: (d) hydrogen tellurate ion.
Ch.2 - Atoms, Molecules, and Ions

Brown15th EditionChemistry: The Central ScienceISBN: 9780137542970Not the one you use?Change textbook
Chapter 2, Problem 113a,b,c,d
Because many ions and compounds have very similar names, there is great potential for confusing them. Write the correct chemical formulas to distinguish between (a) sodium carbonate and sodium bicarbonate, (b) potassium peroxide and potassium oxide, (c) aluminum nitride and aluminum nitrite, (d) iron(II) oxide and iron(III) oxide
 Verified step by step guidance
Verified step by step guidance1
Identify the chemical formula for aluminum nitride: Aluminum nitride is composed of aluminum (Al) and nitride ions (N^{3-}).
Determine the charge of each ion: Aluminum (Al) has a charge of +3, and nitride (N^{3-}) has a charge of -3.
Balance the charges to form a neutral compound: Since both ions have charges that are numerically equal but opposite, one aluminum ion will balance with one nitride ion.
Write the chemical formula for aluminum nitride: The formula is AlN.
Identify the chemical formula for aluminum nitrite: Aluminum nitrite is composed of aluminum (Al) and nitrite ions (NO_2^{-}).

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Ionic Compounds
Ionic compounds are formed from the electrostatic attraction between positively charged cations and negatively charged anions. In the case of aluminum nitride and aluminum nitrite, aluminum acts as the cation, while nitride and nitrite are different anions. Understanding the nature of these compounds is essential for writing their correct chemical formulas.
Recommended video:
Guided course

Ionic Compounds Naming
Nomenclature of Ions
The nomenclature of ions involves the systematic naming of ions based on their composition and charge. Aluminum nitride contains the nitride ion (N³⁻), while aluminum nitrite contains the nitrite ion (NO₂⁻). Recognizing the differences in these ions is crucial for distinguishing between the two compounds and writing their correct formulas.
Recommended video:
Guided course
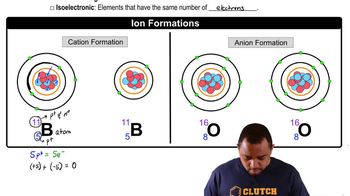
Ion Formation
Chemical Formula Representation
Chemical formulas represent the composition of a compound, indicating the types and numbers of atoms present. For aluminum nitride, the formula is AlN, while for aluminum nitrite, it is Al(NO₂)₃. Understanding how to construct and interpret these formulas is vital for accurately distinguishing between similar compounds.
Recommended video:
Guided course
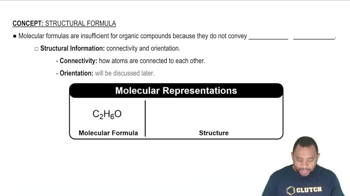
Molecular Formula
Related Practice
Textbook Question
Textbook Question
Many familiar substances have common, unsystematic names. For each of the following, give the correct systematic name: (a) salt peter, KNO3 (b) soda ash, Na2CO3 (c) lime, (d) muriatic acid, HCl, CaO (e) Epsom salts, MgSO4 (f) milk of magnesia, Mg(OH)2.
Textbook Question
Because many ions and compounds have very similar names, there is great potential for confusing them. Write the correct chemical formulas to distinguish between (e) hydride ion and hydroxide ion, (f) magnesium nitride and magnesium nitrite, (g) mercurous chloride and mercuric chloride, (h) cuprous oxide and cupric oxide.
