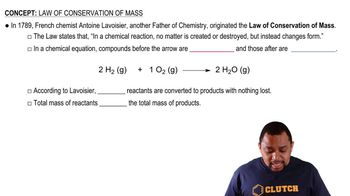
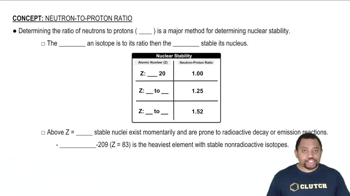
The following diagram represents an ionic compound in which the red spheres represent cations and the blue spheres represent anions. Which of the following compounds is consistent with the drawing?
a. potassium bromide
b. potassium sulfate
c. calcium nitrate
d. iron(III) sulfate